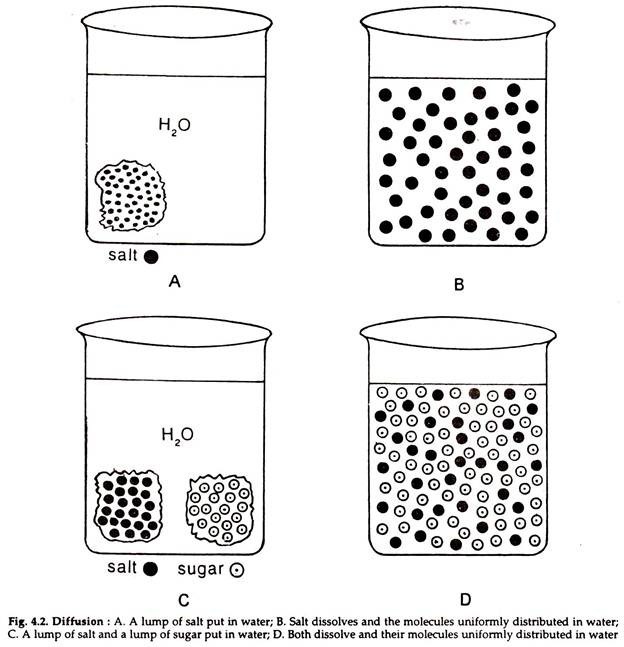
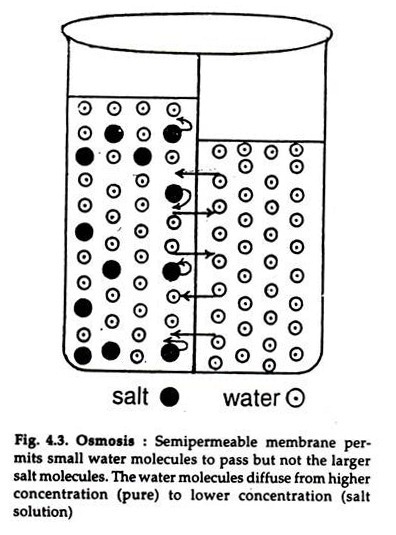
The below mentioned article explains about the osmosis and diffusion with the help of suitable diagrams.
Diffusion:
The movement of molecules of a solute in a solvent from higher to lower concentration is diffusion. The concentration of a solute in a solvent depends on the number of its molecule in a given volume of solvent. The solute molecules move from higher to lower concentration and spread gradually till they are uniformly distributed in the whole solution and an equilibrium is reached.
Molecules in a matter are in constant motion. The movement is a manifestation of the kinetic energy in the molecules. The molecules tend to disperse in a straight line but the direction of movement at any particular moment is unpredictable, because its direction depends on its chance collision with other molecules or the wall of the container (Fig. 4.2).
This retards the rate of movement of the molecules but facilitates the even distribution of the substance in a short period.
The diffusion of the molecules of one substance in a solution is independent of the diffusion of the molecules of other substances. In a mixed solution the relative concentration of each solute is critical in determining the direction of diffusion of the solute. No two molecules can occupy the same space at a time.
Increase in the concentration of one will influence the concentration of the other. In high solute concentration, the solvent concentration must be low. An increase in concentration of one solute results in the corresponding decrease in the concentration of other solutes or the solvent.
Osmosis:
The diffusion of water or a solvent or solute through a semipermeable membrane is osmosis (Gr. osmes = action of pushing). It is passive. When a solution of salt or sugar is separated from water by a semipermeable membrane that allows only the water (small molecules) to pass but not the large molecules of sugar or salt, the movement will be one way, i.e. the water molecules will pass to the solution (Fig. 4.3).
The water molecules diffuse from higher (pure water) to lower concentration (solution). A reverse movement also occurs but at a much slow rate. As a result, the level of the solution rises till an equilibrium is reached, i.e. the weight of the solution exerts a hydrostatic pressure just equal to the pressure of water to enter the solution.
The pressure exerted by the water column is called osmotic pressure. Substances dissolved in the intracellular fluid exerts osmotic pressure on the interstitial fluid and vice versa. When the two processes are equal, there is no entry or exit of water from the cell.
Iso-Osmotic Solution:
A solution in which a cell neither shrinks nor swells, such a solution is called iso-osmotic or isotonic with the intracellular fluid of the cell.
Hyperosmotic Solution:
A cell placed in a solution containing more dissolved substances than in the intracellular fluid, will shrink, as the water from the cell would move out to the solution. Such a solution is known as hyperosmotic with the intracellular fluid of the cell.
Hypo-Osmotic Solution:
ADVERTISEMENTS:
A cell placed in a solution containing less dissolved substances than in the intracellular fluid, swells as the water from the solution moves into the cell. Such a solution is called hypo-osmotic to the cell. Erythrocyte is an ideal material for demonstration of osmosis.
Plasma membrane of R.B.C. is permeable to water but seemingly impermeable to salts. 160 milimolar NaCl solution is iso-osmotic with plasma. If the R.B.C. is placed in a more concentrated (hyperosmotic) solution, water moves out of the cell and the cell shrinks; in iso-osmotic solution water does not move in any way and the cell retains its shape; in a more dilute (hypo-osmotic) solution, the water enters the cell and it swells; water enters the cell when placed in pure water, the cell swells and bursts.