In this article we will discuss about the two phases involved in farming of pearl oysters:- 1. Mother-Oyster Culture 2. Post-Operative Culture.
Phase # 1. Mother-Oyster Culture:
Mother-oyster culture deals with raising of stock by either oyster breeding or by spat collection and rearing them up to a suitable size for nucleus implantation.
A. Pearl Oyster Breeding:
In case of pearl oyster, every mature female produces several millions of eggs, while every mature male produces trillions of sperms. Such high fecundity is provided for the high mortality of eggs, larvae and young ones due to predation and diseases.
ADVERTISEMENTS:
Added to this, is the unpredictable changes in the environmental conditions. All these would reduce the population of oysters to sub-equilibrium levels or even threaten the very existence of the species in that area. Therefore, benign research is needed to rehabilitate the resources.
Formal research led to spawning the oyster under controlled conditions, so as to improve on the chances of survival of the larvae and juveniles (Brooks, 1880). For the Indian pearl oyster, the quest for hatching technology was motivated and formulated by the Central Marine Fisheries Research Institute. It led to the development of hatchery technology for the production of pearl oyster seeds in 1978.
Hatchery Establishment:
The hatchery establishment comprises facilities for good sea water supply, aeration, maturation and spawning of oysters, live algal production for food, larval and spat rearing (Fig. 7.8).
ADVERTISEMENTS:
(a) Supply of Clean Sea Water:
Good quality sea water is to be supplied aplenty as it determines the success of hatchery production of pearl oyster seed. Larvae of pearl oyster are very sensitive to even small amount of pollutants and, therefore, sea water free from pollutants is very essential.
The water drawn from the sea is first passed through sedimentation tanks and filtration units. It is then stored and before use, it is further filtered through a series of cartridges so that all particulate matter of about 3 μm size is removed.
It is further passed through germicidal ultraviolet irradiation to kill bacteria. Optimum range of temperature, salinity and pH should be maintained and any large variation of these parameters would result in failure of hatchery operation.
ADVERTISEMENTS:
(b) Aeration:
Initially larval rearing is done with change of sea water every alternate day. However, for later larval phase, for the spat and for brood-stock maintenance, aeration is required to meet the loss of dissolved oxygen consumption by the larvae, spat and adults.
(c) Maturation and Spawning of Pearl Oysters:
Pearl oysters in a hatchery system have to be maintained in active reproductive phase all throughout the year. This is done by slowly raising the temperature of sea water in which the oysters are maintained from ambient condition to temperatures required for gonad maturation and then for spawning. This practice is common in oyster hatcheries of Canada, USA and Europe.
In tropical countries like India, temperature by itself is not the key factor in controlling the reproductive cycle of pearl oyster, as the annual temperature range from about 25-32°C. However, it has been observed that plentiful food supply provides the energy for reproductive growth and along with a bit of temperature manipulation (within the range of 25-32°C), maturity and spawning of pearl oysters can be obtained.
For production of larvae in the laboratory, both natural spawning and induced spawning are undertaken. Fully mature oysters are then brought from the natural beds or from the farm and placed in the hatchery along with other matured oysters.
The fully mature oyster starts spawning spontaneously and in all cases the male initiate spawning. When this single oyster starts spawning, the chemical substance present in the gametes induces other oysters to follow, resulting in mass spawning in the tank.
However, through physical or chemical stimulation the mature oysters are further induced to spawn at the required time. When pearl oysters are placed in this buffer solution at pH 9.0 for 1-2 hours and then transferred to normal water, they spawn with a success rate of about 78%.
Another chemical stimulation with satisfactory spawning response is obtained when oysters are injected with 0.2 ml of N/10 Ammonium hydroxide at the base of its foot. Mature oysters can also be made to spawn on thermal stimulation when they are maintained at 24°C for a day and then the temperature is raised gradually to 32°C.
ADVERTISEMENTS:
Further, it should be noted that as soon as spawning is noticed, the oysters are transferred from the culture medium to normal sea water so that the eggs and sperms are not subjected to any physiological stress, so as to remain viable at the time of fertilisation.
(d) Production of Live Food:
The veliger larva of pearl oyster measures about 67.5 µm x 52.5 µm and has a mouth opening of minute size. It, thus, requires live food which is smaller than 10 μm in size. Therefore, the dietary requirement of the larvae comprises certain microalgae, particularly flagellates, which are motile, devoid of cell-wall and are not noxious. Thus, in a hatchery system mass production of live algal cells is required to feed the larvae.
Some of the microalgae, generally used as food for the larvae of pearl oysters, is given in Fig. 7.9. Among these Isochrysis galbana and Pavlova lutheri are invaluable. A concentration of 1-2 million cells per ml is given as food to the larvae at appropriate ration. The algae (Fig. 7.9) are mass cultured in 20-litre carboys or in 100-litre perspex tanks.
They are cultured in appropriate nutritional medium under illumination of 1000-1500 lux and photo-period comprising 12 hours light and 12 hours darkness, in cool temperature (23-25°C) and under aseptic conditions. The inoculated cultured stocks multiply and bloom in 4-5 days and are harvested to feed the larvae.
(e) Rearing of Larvae:
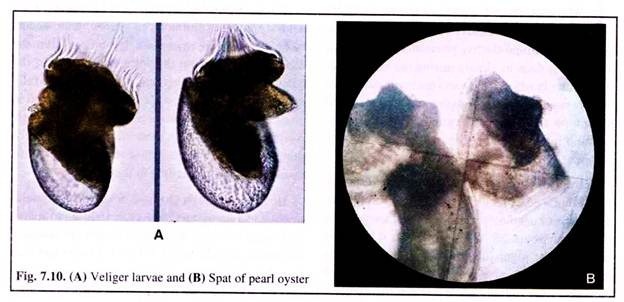
The tiny veliger larvae (Fig. 7.10A) in the hatchery takes about 16-20 days to metamorphose into the adult pearl oyster. Healthy larvae are separated and reared in filtered and sterilised sea water in fibreglass tanks.
They are fed once a day with adequate supplies of microalgal food. The sea water is changed every alternate day after removing the larvae on a sieve. To prevent bacterial and fungal diseases, antibiotics are added to the larval rearing medium.
The veliger larva reaches the umbo stage within 9 to 10 days. The umbo stage larva swims freely and with the development of foot, reaches the pedi- veliger stage. It soon settles down on a substratum. The brief free-swimming period of larval life comes to an abrupt halt.
It then attaches itself to the substratum by secreting the adhesive byssal thread. The larva through a series of morphological changes metamorphoses and attains the shape and form of adult. It attains a size of only 300 μm.
This transformed young oyster is called ‘spat’ (Fig. 7.10B). The spat terminology is used till it reaches a size of about 2 cm shell height. The spat and also the adults discard the bunch of byssal threads, moves a bit away from the original spot by the help of foot and refixes itself on a new spot by secreting fresh threads.
(f) Factors Influencing Larval Rearing:
A number of factors contribute to the success of larval rearing and spat production.
1) Foremost among them is the water quality. Water temperature, pH, salinity and dissolved oxygen should be in the optimum range.
2) The pipeline should be of good P.V.C. material so as to avoid rusting of metal parts. Parallel standby water line should be kept for use when the mainline needs maintenance.
3) The material used in construction of the tank should be such that it does not leach in sea water and should remain clean.
4) Use of detergents for cleaning the tank should be avoided.
5) The hatchery water should be free of dust and insects.
6) The algae food given to larvae should be disease-free.
7) Feeding schedule and ration (quantity) should be precise to avoid decay of excess algae.
Hatching operations should be avoided in seasons when conditions are unfavourable. High mortality rates tend to occur as the larvae are delicate in nature and sensitive to environmental changes. An average of 25% spat production may be considered as satisfactory, although a rate of 50% can be achieved under ideal conditions.
(g) Spat Rearing:
The spat initially are too small and fragile for transplantation to the farm. It needs rearing in the hatchery till it reaches a size of about 3 mm in a month. At this initial phase of spat rearing, good sea water, aeration and adequate amount of external algal food should be provided.
The spat consumes a variety of algae and diatom species. When the spat reaches 3 mm, they are carefully collected from the tanks, placed in fine-meshed velon screen nets with a protective covering and transplanted to the farm.
When the oyster reaches 25 mm, they are taken out and grown in regular baskets. The young oysters fall prey to a number of predators. So areas where prey are abundant should be avoided or these enemies should be periodically removed and destroyed.
B. Spat Collection from Natural Sources And Rearing:
This type of pearl oyster farming deals with raising of stock from natural sources of spat collection and rearing them up to a size (about 20 g in weight) suitable for nucleus implantation.
(a) Farm Site:
The site of pearl oyster farms are selected in sheltered and protected areas of the sea, where the depth should be about 10 m and silting should be minimum. It should be seen that the salinity does not fall below 15 ppt, as this would lead to mortality. Prolonged low salinity condition may occur at the time of heavy precipitation of rain and heavy discharge of rivers in the adjoining area of the farm.
Area rich in phytoplankton should be selected as they are food for the spat. But care should be taken that this bloom is not noxious. A mild water current of 2 knots would help bring in fresh food and at the same time help in removal of metabolic wastes, faecal matters and other farm droppings.
(b) Farming Techniques:
At the onset of culture the technique used was simple, comprising collection of oysters (preferably females) from the natural beds by engaging divers and broadcasting them on the seabed in a protected area near the shore. In such type of practice, the oysters suffered heavy mortality due to covering of silt and sand and also due to predation.
Subsequently, the technique developed was to grow the oysters in the water column which would be well above the bottom. This type of culture came to be known variously, such as off-bottom culture, hanging culture, suspended culture, long-line culture and raft culture. To give the oysters protection from predators, they are placed in nets, cages and baskets.
(i) Nets, Baskets and Cages:
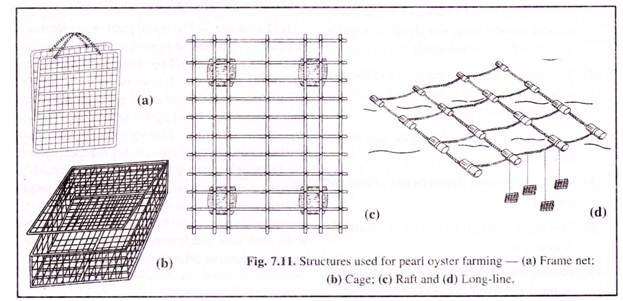
Modern technique of oyster culture has envisaged a change in design and materials of the structures without any change in principle. Pearl oysters are placed in various types of nets, cages or baskets, which are then suspended from the raft or long-line or underwater platforms, by synthetic rope at appropriate depth.
Typical ones used are the frame nets (Fig. 7.11a) or the meshed cages or baskets (Fig. 7.11b). The frame net is useful to avoid crowding of oysters and is easy to follow the post-operative performance of individual oyster. The cage or basket is useful for general mother-oyster culture.
The basket or cages are of various design and made of different materials. Steel rods of 6 mm diameter form the solid frame which is given anticorrosive treatment with paint or plastic coat.
The solid frame is covered with synthetic webbing of appropriate mesh size, so that the oysters do not drop out. A lid to the basket is also made of similar material. The entire structure is periodically checked and required maintenance should be done, so as to increase its life to more than 3-5 years.
The basket or cages have the following structural variations:
(1) Size and shape — square, rectangular or round.
(2) Volume of space — may be flat as in trays and pearl nets or three-dimensional as in baskets or cages.
(3) Number of compartments — the number of compartments in the cages or baskets may be single or multilayered (Fig. 7.11b).
(4) Number of oysters per basket— Oysters are segregated in different size groups and the number of oysters to be kept in a basket varies according to their size. For example, a square basket of 40 cm x 40 cm x 10 cm can hold 125 oysters of 35-45 mm size. 100 oysters of 45-55 mm size, 75 of 55-60 mm size and about 50 in case of larger ones.
(ii) Rafts:
The baskets, cages or frame nets are hung from rafts which are installed in sheltered bays or open sea. Rafts (Fig. 7.11c) are made of wooden poles placed parallel and across, and tied with nylon ropes to form a rigid frame. A standard raft is of 6 m x 5 m size and is convenient in handling.
A couple of planks are fixed on the raft to enable the workers to walk on during inspection. The logs and planks are coated with coal tar. Floats (usually 4 for a standard raft) are attached on the underside of the rafts, so that when it is floated on the sea, the floats remain partially submerged and the poles of the raft remain clear of the water.
Empty air-tight drums (200-litre capacity) with fibreglass coating, mild steel barrels coated with anticorrosive paints, or more modern styrofoam floats or FRP/synthetic floats are used for buoyancy. The choice of float depends on the cost and long-term economics.
The raft after construction on the shore is dragged to the sea and towed by a boat to the selected site of oyster farming. The raft is then anchored to prevent it from drifting away. It is moored by two anchors (grapnel or admiralty type) on opposite sides connected to the raft with tested quality chain.
(iii) Long-Lines:
Long-line culture is best suited for open coastal conditions. Long-lines (Fig. 7.11d) are made of a series of large hollow floats in a row, connected by synthetic rope or chain and suitably anchored. From the strong rope of the line, the baskets containing oysters are suspended and the floats prevent the baskets from sinking.
The lines are flexible and can tide over rough sea conditions. On the other hand, the rafts are rigid and are liable to break under strong winds and waves.
(iv) Underwater Platforms:
For oyster farming in deeper lagoons underwater platforms are used. Oysters in strings are suspended from these platforms.
(v) Choice of Site:
Depending upon the site available, it is important to choose the right technique of farming. In case of calm bays raft culture should be selected, while for turbulent sea long-line culture is ideal. In case of moderately deep lagoons, underwater platforms are best suited.
(c) Environmental Parameters Necessary:
The sea water provides the oysters with all requisites to maintain their life functions. Anthropological fouling of sea water may cause harm to the oysters. Therefore, while selecting the site for oyster farming it is essential to provide the oysters ideal environmental conditions which would ensure success in pearl oyster farming.
(i) Topography:
Pearl oyster farms are constructed in areas sheltered from strong winds and waves. The east and west coasts of India are effected by north-east and southwest monsoons, respectively. As a result, year-round open-sea farming becomes difficult to achieve. Pearl culture in the Gulf of Mannar is restricted to small islands such as Krusadai, Pullivasal, Hare, Karai- challi. Manauli, Nallatannitivu, Vilanguchalli and Vantivu.
In Gulf of Kutch, the ‘Khaddas’ are open intertidal stretches where embankments are erected in the channels to shelter the oyster farm. Moreover, ideal conditions for pearl oyster farms are present in the Andaman and Nicobar Islands and in the lagoons of some islands (Bangaram) of Lakshadweep.
(ii) Hydrographic Conditions:
(1) Depth:
For rearing pearl oyster, depth is an important factor. Quality pearls are produced in deeper waters (beyond 10 m). In the Gulf of Mannar, better quality pearls are produced by oysters in deeper beds (at 15-20 m depths) compared to those produced in intertidal areas. Fouling and boring problems do not occur at depths below 10 m.
(2) Bottom:
Muddy bottom should be avoided. Sea bottom should preferably be gravelly or an admixture of sand.
(3) Water Current:
Water current should be mild (about 1 knot) and would be sufficient to carry dissolved oxygen and food material. Strong current should be avoided as it results in stunted growth of oysters.
(4) Change of Culture Ground:
Repeated culture in the same ground often results in accumulation of biological wastes on the sea bottom, resulting in deteriorative quality of pearl production. Moreover, diseases and mortality of pearl oysters may occur.
(5) Temperature:
The suitable range of temperature is between 18-25°C. During winter when temperature drops to about 10°C, the oysters undergo hibernation. However, in the Indian seas, temperature is high throughout the year and higher growth rate is observed in the winter months. At lower temperatures fine layers of nacre are deposited which improve the lustre of the pearls.
(6) pH:
The pH of the sea water should be between 7-8. In the farm of Tuticorin (presently named Thoothukudi), the annual range of pH is 7.7-8.3.
(7) Salinity:
Salinity in the Gulf of Mannar generally varies between 30 and 35 ppt. Heavy rain in the sea does not bring about any noticeable change in salinity. However, during monsoon heavy discharge of freshwater from rivers, considerably lowers the salinity of costal waters. Prolonged spells of low salinity cause oyster mortality.
(8) Calcium:
Calcium is required for the formation of shell and pearl. Oysters derive calcium from sea water as well as from the body fluids via the mantle. The normal calcium level in sea water is 400 mg/1. In Tuticorin Bay, salinity ranges from 316 to 454 mg/1. Low calcium level would lead to formation of thin shells and poor quality pearls.
(9) Nutrient Salts:
Nutrients such as phosphates, nitrates and silicates are essential for good growth of phytoplankton which forms the food of oysters.
(10) Trace Elements:
The composition of trace elements (quality and quantity) influences the colour of pearls (Table 7.1). Pink coloured pearls contain more sodium and zinc, while copper and silver are responsible for gold- and cream-coloured pearls.
(d) Food of Pearl Oysters:
Several groups of phytoplankton, particularly diatoms and flagellates, form the main food of pearl oysters. The chemical composition and amount of phytoplankton determine the quality of pearl. Blooms of certain diatoms and dinoflagellates sometime result in toxicity and may lead to death of oysters. Therefore, areas which witness such toxic blooms should be avoided for construction of pearl oyster farms.
C. Enemies of Pearl Oyster:
In maintenance of pearl oyster stock, one of the major problems encountered are the enemies of pearl oyster. They can be classified as biofouling, boring and predator organisms.
(a) Biofouling Organisms:
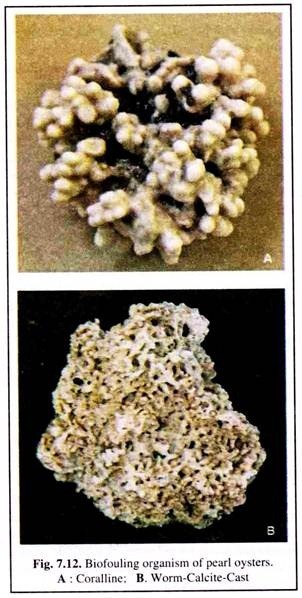
Biofouling organisms settle and grow on the shells of oysters. Several species of marine organisms get a foothold on the oyster shell and form mini-scale biological colonies. These organisms settle as larvae, metamorphose and grow vigorously (Fig. 7.12).
The various biofouling organisms are mainly the barnacles (Balanus amphitrite, whose number may run to a hundred and virtually occupying all the spaces on the shell), bryozoans (Membranipora sp., Thalamoporella sp. and Lagenipora sp.), simple and compound ascidians (Ascidia sp. and Dicarpa sp.), the spat of bivalves (Avicula vexillum and Crasso- strea madrasensis) and hydroids.
Avicula vexillum not only grows profusely on pearl oysters but also on the baskets, almost closing the meshes and disrupting water flow through the baskets. The weaving mussel (Modiolus sp.) forms extensive carpet-like colonies over the pearl oyster beds but causes no serious threat.
Other biofoulers are sea weeds, sponges, amphipods, isopods, polyclad worms, nematodes, opisthobranchiate mollusc, Pinna sp., and egg capsules of gastropods and crinoids.
Biofouling is a major problem in pearl oyster farming as it effects the growth of oyster and the quality of pearls. It necessitates intensive labour work for periodic cleaning of shells. Mechanical devices and water jets are employed to clean oysters and cages. This problem of biofouling can be minimised by choosing deeper waters and selected depths of water, where these fouling organism is relatively less.
(b) Boring Organisms:
The boring organisms riddle through the shells and make the pearl oysters weak and friable. They include two dominant groups — the polychaetes and sponges. The boring molluscs comprising Martesia sp., Lithophaga sp. and isopod Sphaeroma sp. are rarely seen and are of less significance (Fig. 7.13).
Sponges such as Cliona celata, C. vastifica and C. margaritiferae form honeycomb-like ramification in the shell with numerous openings on the nacreous surface.
Polychaets such as Polydora ciliata, Cirratulus cirratus, members of family Syllinidae, Nareidae and Terebellidae cause havoc to the shells. Polydora ciliata cuts tunnels through the length and breadth of the oyster shells.
The oysters, to prevent these predators from creating shell cavity, secretes excess calcium carbonate to seal the entry points. Oysters become weak in the process and in heavy infection may cause large scale mortality.
The boring organisms can be irradicated by placing the pearl oysters in freshwater for about 6 to 10 hours or by application of a thin film of 1% formaldehyde solution on the shells and after few minutes they are again put back in sea water.
(c) Predators:
In farms the major predators of pearl oysters are the gastropods such as Cymatium cingulatum and Murex virgineus, who cause serious damage (kills an oyster per day). These gastropods are seasonal devastators. Crabs such as Charybdis sp., Atergatis sp., Leptodius sp. and Thalamita sp. also prey upon the pearl oysters.
These gastropods and crabs when young (small in size), enters into the baskets through the meshes and grow by preying upon the young pearl oysters. Prevention methods have to be taken through periodic inspection, removal and killing of these predators (Fig. 7.14).
In the natural beds, coral reef perches (Batistes sp, Serranus sp. and Lethrinus sp.), rays (Rhinoptera and Ginglymostotna), starfish and octopus are notorious predators that predate upon pearl oysters.
D. Surgery of Pearl Oyster for Production of Cultured Pearls:
For production of cultured pearl, man provides the oyster with two essential conditions — the core material or nucleus and the mantle tissue. Through skillful surgery, a mantle piece from the donor oyster is grafted into the gonad of the recipient oyster along with a nucleus.
The processes involved are selection of oysters, graft tissue preparation, conditioning graft tissue and nucleus implantation. Later post-operative care is also undertaken.
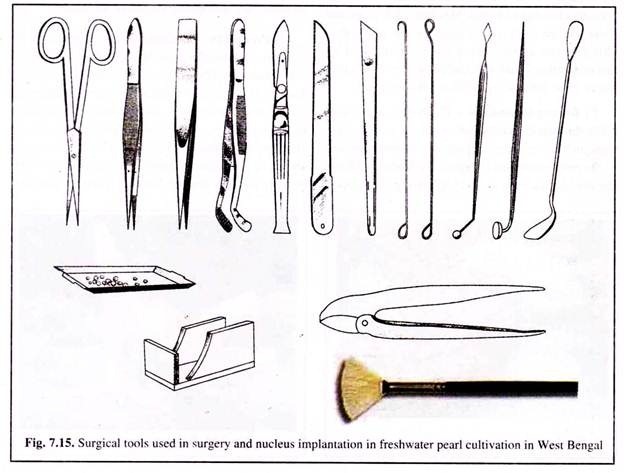
(a) Surgical Tools Used for Surgery:
For pearl oyster surgery, special surgical tools are used (Fig. 7.15). The set comprises oyster knife, speculum, oyster stand with a clamp, graft cutting knife, incising-cum-grafting needle, nucleus implanting needle, needle hook, spatula, scalpel, a pair of scissors and fine hair brushes. The tools are maintained properly after each operation by thorough cleaning with freshwater and drying them in the sun.
(b) Selection of Oysters:
Healthy oysters are selected that are free from any boring and fouling organisms and are free of bacterial and viral diseases. Other factors that are to be considered are the weight and reproductive phase of the oysters. Oysters more than 20 gm of weight are desirable for implantation of nucleus.
Fully matured oysters are not considered. Desirable sized oysters are brought from the farm for surgery and the shells are cleaned of the fouling and boaring organisms. Initial selection is done based on the size and shell quality and the final selection is done only at the time when the oysters are opened for surgery.
(c) Nucleus Preparation:
(i) Raw Material Used:
The core material used for the cultured pearl formation, can be any inert substance. Small Buddha carvings made of ivory, wood, stone or metal castings were used by the Chinese. Mikimoto, the father of pearl culture, used bits of shells, coral pieces, bits of metal, grounded scales of fishes and so on.
Later, since pearl culture was commercialized, it was experimentally obtained that beads of freshwater mussel shells, were the most suitable for use as nuclei for cultural pearls.
The reasons for such conclusion were:
(1) The general physical properties of shell material is similar to that of nacre.
(2) The heat resistant property of shell material and nacre are the same.
(3) The calcium carbonate (in aragonite or calcite form) secreted by the pearl sac, binds homogeneously and firmly with the calcium carbonate layer of the shell bead and, therefore, the pearly component does not get separated from the nucleus at any time.
(4) The density of shell material and nacre are the same. However, in case of blister pearls or half pearls, alabaster is used as the core substance.
(ii) Shell Bead Production:
The shells of various freshwater mussels are used as raw material for production of shell bead. The shell bead manufacturing industry in Japan is highly specialised where the shells from different countries are imported. Here, the shells are cut, ground and processed into spherical shell beads of 2 to 8 mm diameter. They are then supplied to several countries where they are used as nuclei for pearl culture practices.
In India, the sacred chank shell (Xancus pyrum) is used as raw material for nuclei making. From the coastal waters of Tamil Nadu, Kerala and Gujarat, several thousands of Xancus are fished annually and sent to West Bengal, where the cottage shell industry makes bangles and rings from them. However, more than 70% of the shell mass is wasted and often used in making lime.
From these shell waste, thick shell bits are sorted out and then ground into spheres. It is then polished and used as nuclei for cultured pearls. Shell beads are also produced from the shells of giant clam, Tridacna sp., which occur in Andaman and Nicobar Islands. However, freshwater mussels of large size with thick shells are hardly found in India.
(d) Preparation of Graft Tissue:
Graft tissue preparation is essential prior to surgery. The graft tissue is obtained from the mantle of some healthy pearl oysters which are selected as donors. Donor oyster should be in prime condition and without blisters caused by boring organisms. They should not be subjected to any conditioning process (narcotisation, etc.).
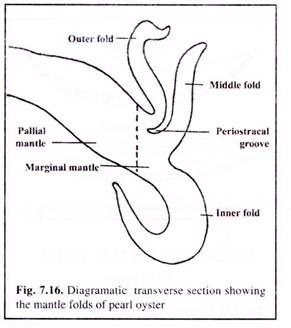
The mantle of pearl oyster is divided into 3 zones (Fig. 7.16) — marginal zone, pallial zone and central zone. It is the pallial mantle that is required for graft tissue preparation as it is more productive in terms of secretion of shell material. The mantle should be removed without injury.
The oyster shell remains tightly shut when taken out of water. It is then cut open by the following method:
(1) The oyster is held dorsal side down and the posterior side facing the person.
(2) The curved end of the sharp oyster knife is inserted between the shells on the posterior side.
(3) The knife is then pushed straight through the oyster until the knife-tip touches the anterior end.
(4) The sharp end of the knife is pressed straight downwards cutting through the adductor muscle.
(5) The valves are opened tearing the hinge, without disturbing the two mantle lobes so that the lobes do not shrink.
The mantle is then removed (taking care that it does not shrink) by the following steps:
(1) The gills are brushed gently with the tip of the spatula, exposing the mantle lobe.
(2) The mantle is then cut with the help of a graft knife, starting from the posterior margin tracing a curve up to the anterior margin.
(3) With the help of forceps, the mantle is gently lifted and the tissue is placed on a soft, clean, moist wooden block. The mantle’s inner epithelium now faces the person.
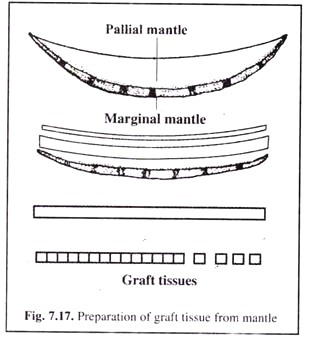
As shown in Fig. 7.17, the graft tissue is further prepared by the following way:
(1) The tissue is first gently stretched end to end.
(2) The mucus and dirt present on the mantle tissue is gently wiped off with the help of a wet sponge.
(3) With the help of a graft knife the marginal mantle (Fig. 7.16), which can be identified due to its folds and pigmentation, is cut off.
(4) Similarly, the inner muscular portion of the mantle on the opposite side is cut off.
(5) With the help of a fresh wet sponge the mucus and dirt are again wiped away.
(6) The mantle ribbon is then lifted by holding one end. The side is reversed and placed on the block such that the outer epithelium faces the person.
(7) Mucus and dirt are again wiped carefully without damaing the outer epithelial layer.
(8) The margins of the mantle are further trimmed on either side until a ribbon of about 3 mm width is obtained.
(9) All the dirt and mucus are wiped off from the wooden block and if necessary, the wooden block may be changed. Care should be taken while transferring the mantle ribbon that the sides are not changed.
(10) With the help of the graft knife, the ribbon is cut into small pieces (about 2-3 mm) in proportion to the size of nucleus.
(11) The small pieces of the graft tissue is smeared with a very dilute solution of water soluble eosin by the help of a brush.
(12) Care should be taken such that the tissues remain moist until they are used within 30 minutes.
Precautionary methods to be taken are:
1) Care should be taken such that the oyster knife while inserting between the valves does not slip and injure the palm of the person.
2) Clean, filtered sea water should be used during the entire operation.
3) All the instruments should be sterilised and dried in sunlight prior using.
4) Clean and moist sponges should be used and should be changed after each wiping of mucus and dirt.
5) Wooden blocks must be smooth, clean and should be moist till the graft tissues are used up.
6) The shrunk mantle lobes (due to bad handling) should not be used.
7) The tissues, generally should be used up preferably within 10-15 minutes of preparation. Retention of tissue on the block for a long time leads to deterioration and failure in pearl sac formation.
(e) Conditioning of Oysters Prior to Surgery:
Conditioning of oysters are generally undertaken before surgery. It is either done naturally or by chemical.
(i) Natural Physical Conditioning:
Such methods are ideal and inexpensive. It can, however, put into use in regions where there is stratification of temperature in the sea (as in Japan) and sharp difference in food availability. By using the thermal difference the oysters are made to spawn in higher temperature of the surface water.
The loss of stored energy thus encountered due to spawning, weakens the oysters. They are then further subjected to starvation stress in depths of low phytoplankton production. This reduces the metabolic rate.
(ii) Chemical Conditioning:
In Indian waters the above technique does not work. So the oysters are resorted to chemical conditioning. Oysters are first put in tubs placed in sea water. Over it menthol crystals are then sprinkled. Under the effect of menthol, the oysters get narcotised in about 60-90 minutes. The adductor muscle of the oysters relaxes and the valves open.
The response time varies with temperature and the oysters become almost non-responsive to touch. One by one they are taken out of the tub, washed in sea water and then used in surgery. Pearl oysters should be treated with menthol in batches as prolonged exposure in this chemical causes swelling of tissues, large secretion of mucous and ultimate mortality. Safe limit of exposure in menthol is about 30-45 minutes.
(f) Implantation of Nucleus:
(i) Nucleus Implantation for Round Pearls:
(1) Site of Implantation:
The site of formation of natural pearls in oysters is the muscular region mainly in the pallial connective tissue, tissues around the liver and kidney, levator muscle and in the palpar region. The pearls formed are generally small in size and irregular in shape.
In case of cultured pearls the technology aims at producing pearls of large size and of smooth, round shape. For implantation of nucleus, the region of the body to be selected should have adequate space for accommodation of large sized nucleus and the organ should not be highly specialised.
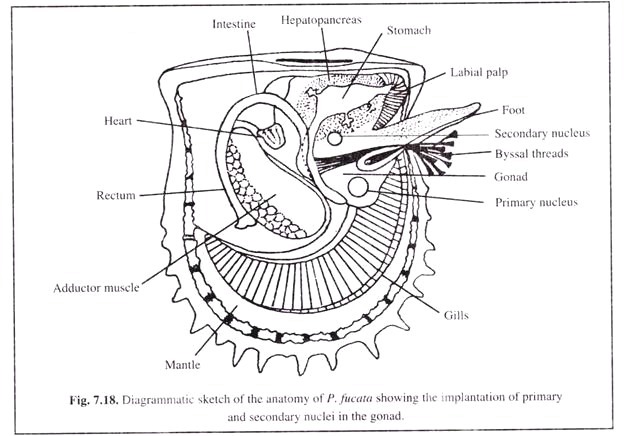
Thus, the gonadal region of pearl oysters has been found to be the ideal site (Fig. 7.18) and the follicles carrying the gametes get reorganised without greatly affecting the reproductive function of the oyster.
As a secondary site, the peripheral region near the liver or hepatqpancreas is also used for nucleus implantation, but care should be taken so that the stomach, intestine and other vital organs such as heart, kidney, etc. are not damaged.
(2) Number of Nucleus Implanted:
In oysters of sufficiently large size, a maximum of 5-6 nuclei of 4 mm diameter or less can be implanted. Single nucleus implantation is done for nucleus of large size (6 mm diameter). For double nuclei implantation, 2 nuclei of 6 mm and 5 mm diameters are used, while nuclei of 4 mm or less diameter are used for triple and higher nucleus implantation.
The nucleus load also depends upon the size of oyster and condition of gonad. Generally, in case of two-nuclei implantation, the primary nucleus (larger one of the two) is implanted in the gonad at its ventral extremity, while the smaller nucleus (secondary nucleus) is implanted at the dorsal side close to the hepatopancreas (Fig. 7.18).
(3) Oyster Surgery Conducted:
Pearl oyster surgery is done by the following steps:
i. The conditioned oyster is first mounted on the stand and positioned between the two plates of a clamp in a manner to suit the convenience of the technician and also that it does not slip out.
ii. The end of the speculum is inserted into the posterio-ventral corner of the oyster in between the two valves, and into the opening is placed the gap- regulator ring.
iii. With the help of a spatula the mantle, gills and labial palps are smoothly pushed aside to get a clean view of the gonad region.
iv. The tip of the foot is hooked with the needle hook and is gently lifted so that the base of the foot is slightly elevated. The needle is held in position till the operation is completed.
v. With the end of the oval knife, a sharp incision of length sub-equal to the diameter of the primary nucleus, is made in the pigmented region at the base of the foot. The muscles are gently detached with the sharp oval end of the needle and a passage is cut through the gonad up to the site of implantation. The needle is then gently withdrawn.
vi. A piece of the graft tissue (Fig. 17.17), present on the block is picked with the help of the reverse side of the same needle (as used before). The mantle piece is then inserted gently through the passage cut in the gonad and left at the site of implantation. The needle is then withdrawn.
vii. The nucleus is lifted by the appropriate nucleus- implanting needle with the help of the cup-end. The nucleus is inserted gently through the incision made previously at the foot and the passage cut through the gonad. On reaching the site, the nucleus is dropped by deflecting the needle.
The nucleus comes in contact with the outer epithelium of the mantle tissue, which was previously grafted into the gonad. The outer epithelial side of the mantle piece should face the nucleus and this orientation is important for successful formation of the future pearl sac. The needle then is gently withdrawn through the passage.
viii. The two margins of the incision are then allowed to come in contact in the pigmented region.
ix. The oyster is then removed from the clamp, the speculum is withdrawn and the gap-regulatory ring is slipped out. The oyster is then placed in fresh sea water.
x. In case of multiple implantation, the process is repeated for each nucleus implantation which is done through the same incision.
(4) Precautions to be undertaken:
i. The instruments should be cleaned in sea water before and immediately after use.
ii. Care should be taken such that the valves are not opened too much, so that the adductor muscle snaps and the oyster dies.
iii. The pressure on the foot should be adjusted in a manner so that the foot does not get torn while pulling it with the needle hook.
iv. The incision made on the foot region should be a sharp cut and should be just of required length as the size of the nucleus to be implanted. If the incision made is too large and if much tear of tissues has taken place then it is advisable not to proceed further and the oyster be placed back in fresh sea water to be taken back to the farm.
v. If there is copious flow of gametes at the time of cutting a passage through the gonad, then it is advisable not to proceed further and the oyster should be taken back to the farm with the general stock.
vi. At the time of surgery care should be taken not to damage the vital organs such as stomach, intestine and heart.
vii. The orientation of the graft tissue and nucleus should be proper, i.e., the nucleus should be in contact with the outer epithelium of the mantle tissue.
viii. Perfection in the surgery should be undertaken with skill and patience.
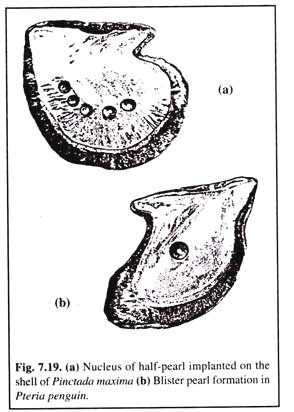
(ii) Half-Round Pearl:
In P. maxima, P. margaritifera and the winged oyster Pteria penguin, half- round pearl is produced using the pearl-Buddha technique of China. In this case the nuclei implanted ranges from 10 to 20 mm in diameter and is made of alabaster.
The technique adopted is as follows:
(1) The oyster is first clamped horizontally on the stand, and from the upper valve, the mantle is separated.
(2) A drop of ‘aron-alfa’ (a special transparent adhesive) is applied to the shell.
(3) The nucleus is pressed against the adhesive for 2 minutes by a ring.
(4) By this method, the required number of nuclei are fixed to the shell (Fig. 7.19).
(5) The oyster is then turned over and the opposite shell is treated in the same manner, taking care that the nuclei on both shells do not oppose each other.
(6) The number of nuclei fixed in each shell depends upon the size of the nuclei — 3 of 16-17 mm diameter and 5 of 13 mm diameter.
Phase # 2. Post-Operative Culture:
The stress endured by the oysters due to surgery and conditioning makes them weak and vulnerable. Careful handling is essential until the oysters recover fully. The operated oysters, thus, should be placed in gently flowing sea water or in a static system with frequent change of sea water.
In case of a bay or lagoon, where the sea water is transparent, the oysters can be caged and directly suspended from the rafts. It is, however, advisable that the operated oysters be kept for 3-4 days in the surgery facility under observation before they are returned to the farm.
The oyster, on being placed in sea water, would, start shutting the valves within a few minutes as the effect of menthol gets over slowly and would sooner resume its normal function of opening and shutting the valves.
Normally the incision would heal within 2-3 days. At this time, the oyster would attempt to get rid of the nuclei. If the incision and the passage cut through the gonad are too large then the chances of the nucleus getting rejected are greater.
Also, rough handling and exposure to strong stimuli (tides, current, large waves and food supply) would also result in rejection of nuclei. In case of oysters whose vital organs have been damaged during surgery, would suffer mortality.
In case of single, large nuclei implantation, the farmer has to be sure that the nucleus has not been rejected by the oyster, for in that case, he would be culturing the oyster containing no pearl over a long period of culture duration.
Such oysters implanted with 7-8 mm diameter nucleus are checked by X-ray to make sure of nucleus retention. P. maxima are X-rayed after 3-4 months of post-operative care. It has been noticed that 83% of these oysters retain the nucleus. Those oysters who have rejected the nuclei are shifted back to mother-oyster culture and are later re-operated or used for half-pearl culture.
Pearl oysters who have successfully gone through the post-operative care period are put in baskets or cages and further reared in the rafts in deeper waters (than the mother-oysters). These oysters are left undisturbed and are cleaned from time to time (once in a quarter) depending upon the load of fouling organisms.
The density of oysters per basket is reduced to 50-60% of the load of mother-oyster culture, so as to provide better growing conditions for each oyster. The baskets are suspended in areas of high phytoplankton population.
The duration of post-operative culture depends upon the time required for the pearls to attain lustre and thickness of the nacreous layers. The duration of the post-operative culture in Japan has been progressively reduced from 2 ½ years in 1960 to 6-8 months in recent years.
This is due to commercial compulsion. Ward (1985) has stated that a nacre thickness of 0.5 mm is acceptable as pearl and the minimum is 0.35 mm for good lustre and colour. However, the thickness obtained in 6-8 months postoperative culture is 0.2 mm.
In India, under tropical conditions, the acceptable thickness for good lustre and colour are produced within 3-4 months with nuclei of 2-3 mm diameter and in 15-18 months with nuclei of 6-7 mm diameter.
A. Influence of Temperature on Nacre Formation:
Laying down of nacre around the nucleus is influenced by the temperature of sea water. In Japan, the temperature of waters in winter goes down to as low as 10°C. At this temperature, the metabolic activity of the oysters is very low and they enter into a state of hibernation. At this period, the nacre secretion is practically arrested. Nacre secretion recommences only when the temperature rises in spring.
In India, high temperature prevails throughout the year and hence nacre secretion is uninterrupted and hence the growth of pearl is faster.
B. Harvest of Pearls:
Harvesting of pearls, also known as ‘beaching’ of pearls, is done during period of low temperature and pH. The cooler period of the year is selected as the lamellae of nacre layed down at this period are thin.
This thin lamellae on the top are considered to be best for colour and lustre. Generally, the farmers at the end of post-operative culture, shift the oysters to grounds which favour production of pearls of superior quality. This phase is known as ‘make-up’ culture.
The harvested oysters are taken up individually and opened to collect the pearls. P.fucata oysters are cut open with a sharp knife to take out the pearls and thereby killing the oyster.
However, in case of P. maxima, the oysters are opened carefully taking care not to kill the oysters at the time of removal of the pearls. These oysters are then reused for at least one more crop by inserting a new nucleus of appropriate size in the already formed pearl sac, without using a fresh mantle piece.
In case of old aged oysters, they may contain some natural pearls, besides the cultured ones. After removal of the cultured pearls (from these oysters), the flesh is separated from the shell and soaked in slack lime.
They are then placed in a wooden vat fitted with rotating wooden blades. The flesh gets pounded into a slurry. Due to the higher density, the pearls settle at the bottom of the vats, which are then collected, washed with neutral soap water and dried.
C. Gross Production:
Some Oysters during post-operative culture period suffer mortality due to natural as well as surgical causes. It has been estimated that 20-30% of operated P. maxima die. It has also been seen that 10-15% of operated oysters reject the nucleus even under the most healthy post-operative conditions.
Pearl yield is calculated based on the weight of pearl produced from 10,000 invested nuclei. Generally 10,000 numbers of 6 mm nuclei produce 6.75 kg of pearls for a gross yield of 70%. While 7 mm nuclei produce 9.38 kg of pearls at a gross yield of only 40% as the mortality rate is much higher.
Recent development and future trends in Pearl Culture:
Pearl culture is perceptibly changing from a monopolistic industry to a trend of diversification in several parts of the world. Success of this trend depends on development of appropriate technologies which would lead to the production of quality pearls in different species of oysters in different regions.
The trends and developments are:
(a) Science and Technology Trends:
The future of pearl culture would depend upon the quality rather than quantity of pearl production.
(b) Resource Management:
Oysters are sedentary organisms having restricted distributions and thrive only under certain ecological conditions.
So judicious management is required as fishing pressure and pollution is taking its toll resulting in degeneration of stock. Sea-ranching in recent years has been adopted as a technique of improving the natural stock of oysters.
(c) Improvement of Mother-Oyster Stock:
Hatchery production of pearl oyster is widely followed to replenish the dwindling stock. Attempts now has centred in identifying the genetic characteristics of a few isolated wild stock and in building a breeding programme on this material.
By selective breeding, pearls without yellow pigments can be more effectively produced. Genetic work in pearl oyster has been directed towards producing stocks yielding pearls of desirable quality, resistant to microbial diseases, adaptable to environmental stress and having high growth potential.
Mass production of P. fucata in India has been established through hatchery production. Selective breeding of P. fucata has been undertaken with regard to colour and quality of nacre.
(d) Pathological Problems:
As oysters are reared in limited areas in high density, there is every chance that diseases would infest and spread fast. This would lead to excessive mortality of oysters (80%) resulting in serious impact upon the industry.
The main culprit being marine Vibrio bacteria, and control of the disease outbreak becomes impossible. Identification of the pathogen and prophylactic measures should be undertaken. Intensive research in this field is of top priority to safeguard the farm stock.
(e) Environment of Pearl Culture:
The quality of pearls produced is influenced by the environmental factors. This has led to the establishment of farms and shifting of culture grounds to conducive environmental areas. However, further investigations are needed to understand the critical aspects of environment and their influence on the physiological function of secretion of the organic and inorganic components of the pearl.
The factors that plays a vital role in the colour and texture of pearl and better secretion of nacre has been discussed earlier. If these factors can be artificially extra polated in pearl farming then both better quality and higher quantity of pearls can be produced. These will be areas of future research.
(f) Techniques of Surgery:
In the past few years great improvement has been achieved in producing black pearls in P. margaritifera.
The future challenges to be encountered are:
(1) to control the mortality of oysters in the post-operative phase,
(2) reduce the rate of rejection of nuclei,
(3) improve production of quality pearls,
(4) to increase the size of the nuclei up to 16 mm diameter and
(5) production of round nucleated pearls having size up to 17 mm diameter, in freshwater mussels.
(g) Bio-Mineralisation and Spectral Characteristics of Nacre:
Mineralisation and spectral characteristics of pearl have been the subject of research to understand the formation of organic matrix and crystalline microlayers. A sound knowledge on these is required which would help in controlling the quality of pearls and these may lead to new dimensions in the future.
(h) Tissue Culture:
Culturing of pearl oyster mantle tissue has been persued for many years. From such tissue culture experiments, it has now been possible to produce pearls by injecting around the implanted nucleus a fraction of the cell suspending liquid obtained from mantle epithelial cell culture.
The cells in suspension forms the pearl sac which would secrete nacre leading to pearl formation. If the type of epithelial cells that would be secreting the aragonite crystalline matter, can be isolated, then it might be possible to culture the finest pearls in vitro, outside the body of pearl oyster.
(i) Farming Technology:
To make farming of pearl oyster cost-effective and less fatiguable for people working in the farms, it is essential to modify and improve the system, design and materials of farming.
(j) Organisational Set-Up:
Pearl industry over the days have become a composite structure that comprises raising of mother-oyster, pearl production, processing and marketing. All these fields have become highly specialised requiring separate technology, skill, capital, manpower and equipment.
The advantage is that it can mobilise resources, manage problems with competence and can have greater control over situation. However, the disadvantage is that, if there is any miscalculation in any of the areas, then it can upset the entire system.
(k) Formation of Pearl Farmers’ Co-operative Society:
Young farmers with expertise for pearl culture may organise themselves into a Pearl Farmers Co-operative Society. The society with state financial support will look after input supply as well as output management.
(i) Input supply — It includes the following:
(1) Facilitation of leasing sea area and shore facility.
(2) Supply of oysters.
(3) Supply of farm materials.
(4) Supply of nuclei, chemicals and equipment’s.
(5) Security arrangements.
(6) Members training arrangements.
(7) Spat supply arrangements with Government organisations (in the initial stages). Collection from the natural beds to be kept as secondary option in seasons of abundance.
(8) Import of shell beads (in the beginning). The society would later set up its own unit.
(9) Society should have its own hatchery in course of time.
(ii) Output Management — The society will look after the following:
(1) Purchase the pearls produced by the individual units.
(2) Help in sorting, grading and processing.
(3) Sell the pearls in the domestic market and export them.
(4) The by-products to be properly utilised.
Policy Formation:
Employment generating potential is present in pearl culture and it provides employment for;
(i) skilled and semi-skilled persons in the coastal rural areas and
(ii) managerial and technical personnel.
Pearl adds to the GNP and helps in earning foreign exchange. It is a new industry and pearl culture is a long-term investment, so it will need policy support, financial assistance, subsidy aid economic incentive from the Government in the early stages, till it becomes self-sufficient.