All molecules of biological importance, except water, carbohydrates and fats, contain nitrogen. Proteins, amino acids, chlorophyll etc. all contain nitrogen. Of total body dry weight, nitrogen makes up almost 13%, which is the third-most abundant element after carbon (48%) and oxygen (24%).
The atmosphere contains approximately 78 per-cent nitrogen, mostly in gaseous form (N2), and thus forms the greatest reservoir and safety valve of the system. However, this large reservoir of nitrogen in the air is not directly available to plants, as the strong triple bond of atmospheric nitrogen makes it inaccessible.
Nitrogen becomes accessible to organisms through biological transformation of gaseous nitrogen into nitrate (NO−3) and ammonium (NH3). This form of nitrogen is referred to as ‘fixed’ nitrogen as it can now enter biological processes. Most plants, thus, take in nitrogen as nitrate or ammonia. Animals produce their proteins from food, while decomposers make it from dead animal and plant tissues.
During respiration, when proteins are broken down, a waste product containing nitrogen is produced. Some organisms excrete ammonia directly as the usual cellular waste, while many convert it to less toxic compounds.
ADVERTISEMENTS:
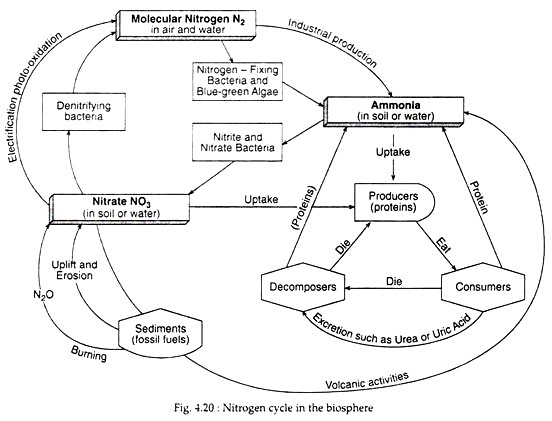
Mammals convert ammonia to urea or uric acid. Such compounds are broken down to ammonia by decomposers to obtain energy. The entire process of nitrogen cycle, as given in Fig. 4.20, can be summarised as shown:
ADVERTISEMENTS:
Nitrogen Fixation:
In the nitrogen cycle, molecular nitrogen enters the biological pathways primarily through an assimilation by certain microorganisms, particularly bacteria, and also through lightning. Such a process is referred to as nitrogen fixation.
Nitrogen fixation is carried out by certain species of bacteria and cyanobacteria in both aerobic and anaerobic environments. Some are free-living, occurring in soil or in water, while others exist in symbiotic relationships with higher plants.
The most well-known symbiotic nitrogen fixation is done by Rhizobium bacteria that live in the root nodules (Fig. 4.21) of many plants in the family Leguminosae (peas, groundnuts, beans, clovers etc.). It is a symbiotic relationship where the legume gets a plentiful supply of ammonia from the bacterium (for protein production), while the bacterium receives photosynthetic and a suitable microhabitat.
Nodules are also present in various non-legume trees and shrubs, such as alders, where the nitrogen-fixing organisms are the actinomycetes. Aquatic symbiosis is witnessed between the aquatic fern Azolla and the nitrogen-fixing cyanophyte Anabaena (blue-green algae).
Anabaena lives in pores in the fern fronds. Many grasses have nitrogen-fixing bacteria associated with their roots in a region called rhizosphere, a transition zone between the root and the soil. Fixation rates are, however, much less in the grasses than for species that possess root nodules.
Non-symbiotic nitrogen fixation is seen in the case of free-living cyanophytes and bacteria in soil and water, and also by ones living epiphytically on trees and other plants. Nodules are formed when Rhizobium infect a root hair or damages epidermal cell. The root nodules contain tetraploid host cells with the bacteria and some diploid cells without the bacteria.
The root nodules contain leghaemoglobin, that functions in much the same way as our own haemoglobin in transporting oxygen. Leghaemoglobin controls the rate at which oxygen reaches the nitrogen-fixing bacteria, as too much oxygen inactivates the enzyme that catalysis nitrogen fixation. Nitrogenase is the enzyme responsible for nitrogen fixation.
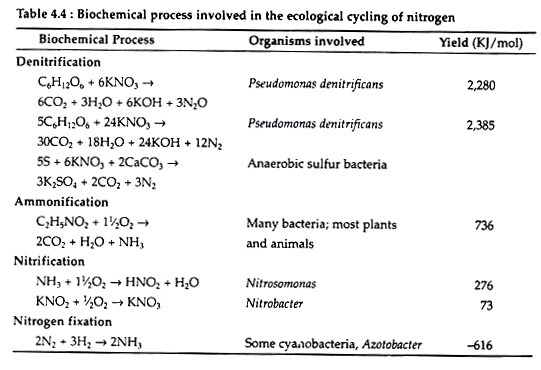
The reason why few organisms make direct use of the nitrogen in the atmosphere is because nitrogen fixation is an expensive process energetically (Table 4.4). Nitrogen molecule (N2) being very stable, to fix one molecule of it, requires 16 molecules of ATP.
Not only this, it also requires a number of enzymes. Klebsiella pneumoniae, a soil microorganism, has a total of 17 genes called nif genes, responsible for making the polypeptide involved in nitrogen fixation.
Ammonification:
Once in the biological realm, the first step in the nitrogen cycle is ammonification—a process that involves the hydrolysis of protein and oxidation of amino acids, resulting in the production of ammonia (NH3). This transformation is carried out by all organisms, where during the initial breakdown of amino acids, energy is released (Table 4.4). Ammonia is retained in the soil (as ammonium ion) which the plants can use directly. The basic cycle is completed with the excretion of ammonia by consumers and decomposers (Fig. 4.20).
ADVERTISEMENTS:
Nitrification:
Ammonium ions (NH4+) are oxidised within a few days of their formation or addition as a fertiliser. Nitrification involves the oxidation of nitrogen, first from ammonia to nitrite and then to nitrate:
NH3 → NO−2 (N3− →N3+)
NO2 → NO3– (N3+ → N5+)
During this oxidation, nitrogen atom releases much of its potential chemical energy (Table 4.4), Both the above steps are carried out by specialised bacteria— Ammonia to nitrite by Nitrosomonas in the soil and Nitrosococcus in marine systems, while nitrite is converted to nitrate by Nitrobacter in the soil and Nitrococcus in the seas.
De-Nitrification:
During nitrification the oxidation of nitrogen requires the presence of oxygen to act as an electron acceptor. In oxygen depleted bottom waters or waterlogged anoxic soils and sediments, nitrate and nitrite can act as electron acceptors (oxidizers) resulting in the reversal of nitrification reactions, called de-nitrification:
NO3 → NO2 → NO
(N5+ → N3+ → N2+)
Nitrogen monoxide is then converted to di-nitrogen oxide and then to molecular nitrogen
NO N2O N2
Thus, de-nitrification involves the reduction of the nitrate ion (NO−3) to nitrogen. This is accomplished by certain anaerobic bacteria (Pseudomonas denitrificans) which have the ability to use the nitrate ion as an electron acceptor in respiration. Consequently, the loss of nitrogen from biological circulation takes place. This may be one of the major causes of the low availability of nitrogen in marine systems.