The following points highlight the top four mechanisms of prevention of blood loss. The mechanisms are: 1. Vascular Spasm 2. Formation of the Platelet Plug 3. Blood Clotting and Coagulation 4. Growth of Fibrous Tissues into the Blood Clot to Close the Hole.
Mechanism # 1. Vascular Spasm:
The wall of the blood vessel contracts, immediately after a cut or rupture of the vessel. This instantly reduces the flow of blood from the ruptured vessel. The contraction results from both nervous reflexes and local myogenic spasm. This local vascular spasm lasts for 20 to 30 minutes. During this period the processes of platelet plugging and blood coagulation take place.
ADVERTISEMENTS:
Mechanism # 2. Formation of the Platelet Plug:
This is the second phase of haemostasis, where platelets come in action. When platelets reach the damaged vascular surface, such as the collagen fibers in the wall or even the damaged endothelial cells, they immediately change their characteristics.
They begin to swell, assume irregular forms with numerous irradiating processes protruding from their surfaces. They become sticky and stick to the collagen fibers, secrete ADP and enzyme thrombin. Then activate other platelets and ultimately numerous platelets accumulate in the split or damaged part of the vessel to form platelet plug.
The platelet plug itself can stop blood loss completely, if the severed vessel is small. Bui, if there is a large hole, a blood clot in addition to the platelet plug is required to stop the bleeding.
Mechanism # 3. Blood Clotting and Coagulation:
ADVERTISEMENTS:
Coagulation is a complex sequence of chemical reactions which results in the deposition of insoluble fibrous proteins to form a clot. The clot is composed of a meshwork of fibrin threads running in all directions and entrapping blood cells, platelets and plasma. The fibrin threads adhere to damaged surfaces of blood vessels. Therefore, the blood clot becomes adherent to any vascular opening and thereby prevents blood loss.
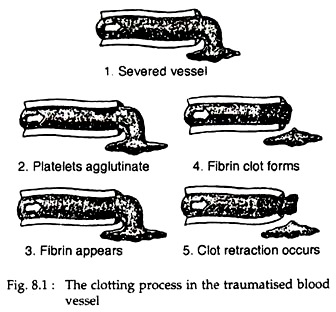
The process of formation of blood clot is called clotting. This is the third mechanism for haemostasis. The clot starts to develop within 15 to 20 seconds, in case of larger injury and within one to two minutes in smaller injuries. The physical events in this process are described in Fig. 8.1. The chemical events are described here.
Almost all research workers in the field of blood coagulation agree that clotting takes place in the following essential steps:
ADVERTISEMENTS:
A. A substance or complex of substances called prothrombin activator is formed in response to rupture of the vessel or damage to the blood itself.
B. Prothrombin activator catalyses the conversion of prothrombin to active thrombin.
C. Thrombin acts as an enzyme to convert fibrinogen into fibrin threads that enmesh platelets, blood cells and plasma to form the clot itself.
D. Partial or complete dissolution of the haemostatic plug or thrombus occurs by plasmin.
Varieties of Thrombi:
There are three types of thrombi or clots, which can be distinguished by the variable proportion of fibrin in them.
(a) The white thrombus is composed of platelets and fibrin and is relatively poor in erythrocytes. It forms at the areas where blood flow is rapid, e.g., arteries.
(b) The red thrombus consists primarily of red cells and fibrin. It forms in areas of retarded blood flow or stasis (e.g. veins) with or without vascular injury.
(c) The third type is a disseminated fibrin deposit in very small blood vessels or capillaries.
ADVERTISEMENTS:
Coagulation chemistry:
Fibrin formation:
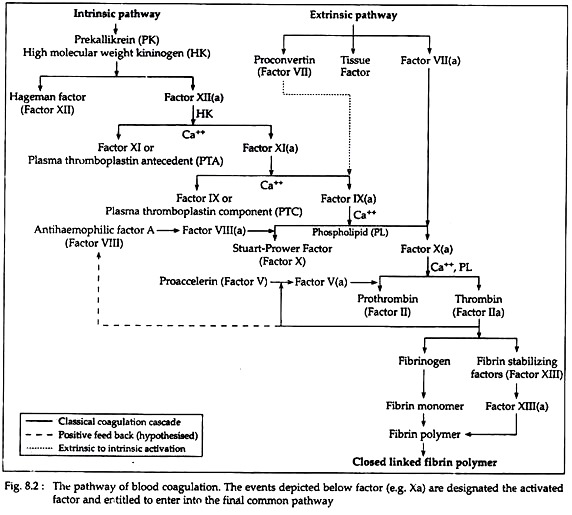
There are two pathways involved in the fibrin clot formation— the intrinsic and the extrinsic pathways. In intrinsic pathway the initial factors originate from within the blood, whereas in extrinsic pathway initial factors are found from the injured tissues. However, these pathways are not independent, as previously thought.
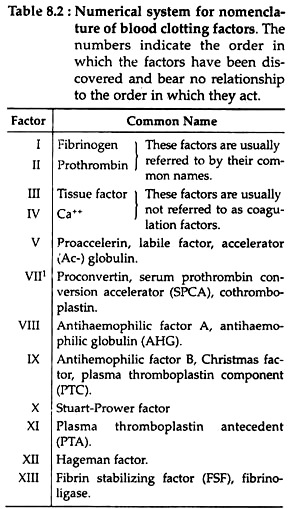
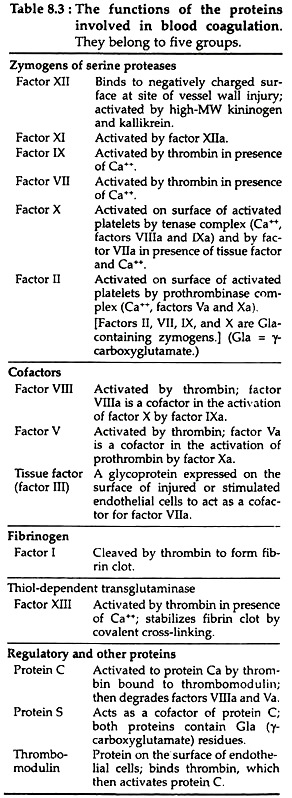
After initial activities, both pathways converge in a final common pathway. The intrinsic, extrinsic and final common pathways are complex and involve many different proteins (Fig. 8.2 and Table 8.2). Functionally these proteins can be classified into five types (Table 8.3):
Activation of Factor-X (Stuart-Prower factor):
A. By intrinsic pathway:
The intrinsic pathway (Fig. 8.2) involves factors XII, XI, VIII and X (Table 8.2) as well as prekallikrein (PK), high molecular weight kininogen (HK), Ca++ and platelet phospholipids (PL). This result in the formation of factor Xa (i.e. activated Stuart-Prower factor). By convention, activated clotting factors are referred to by use of the suffix a.
The pathway is described in the following steps:
(i) Activation of factor XII (Hageman factor):
The intrinsic pathway commences with the ‘contact phase’ in which PK, HK, factor XII and factor XI are exposed to a negatively charged activating surface (Fig. 8.2). The factor XII is activated to factor Xlla upon proteolysis by kallikrein. This factor Xlla attacks PKs to generate more kallikrein.
(ii) Activation of factor XI (PTA = plasma thromboplastin antecedent):
The kallikrein, generated from factor XII- activation, activates factor XI to produce XIa and also releases bradykinin from HK. Bradykinin is a nonapeptide with potent vasodilator action.
(iii) Activation of factor IX (PTC = plasma thromboplastin component):
Factor XIa in the presence of Ca++ activates factor IX to the serine protease factor IXa.
(iv) Activation of factor X (Stuart-Prower factor):
This in turn cleaves factor X to produce the two chain serine protease and factor Xa. For the completion of this reaction it requires the assembly of components, called the tenase complex, on the surface of the activated platelets. The tenase complex contains Ca++, factor Villa, factors IXa and X.
For assembly of tenase complex, the platelets must first be activated to expose the acidic phospholipids, phosphatidylserine and phosphatidylinositol, that are normally on the internal side of the plasma membrane of resting, non-activated platelets.
Factor VIII is a cofactor that serves as a receptor for factors IXa and X on the platelet surface. Factor VIII is activated by minute quantity of thrombin to form factor Villa, which in turn is inactivated upon further cleavage by thrombin.
B. By extrinsic pathway:
This pathway is triggered with blood coming in contact with traumatised vascular wall or extravascular tissues. This pathway is completed in following three steps (Fig. 8.2).
(i) Release of tissue factor and tissue phospholipids (PL):
The traumatised tissue releases two factors that set the clotting process in motion. These are (a) tissue factor, which is a proteolytic enzyme, and (b) tissue phospholipid, which are mainly phospholipids of the tissue cell membranes.
(ii) Activation of factor VII (SPCA = serum prothrombin conversion accelator/Proconvertin):
The tissue factor complexes with factor VII and enzymatically converts it to factor Vila.
(iii) Activation of factor X (Stuart-Prower factor):
Factor Vila cleaves factor X to form the two-chain serine protease (i.e., factor Xa) by its enzymatic activity.
Final common pathway for clotting:
(i) Activation of prothrombin (Factor II):
In this pathway, factor Xa, produced by either intrinsic or extrinsic pathway, activates prothrombin (factor II) to factor Ha (Thrombin). Thrombin then converts fibrinogen to fibrin (Fig. 8.2). The activation of prothrombin occurs on the surface of activated platelets, and requires the assembly of prothrombinase complex. This complex consists of platelet anionic phospholipids, Ca++, factor Va, factor Xa and prothrombin.
(ii) Role and activation of factor V (Proaccelarin):
Factor V (proaccelarin) is a glycoprotein and acts as a cofactor in a similar manner to that of factor VIII in the tenase complex. It is activated by traces of thrombin and binds to specific receptors on the platelet membrane and forms the prothrombinase complex. Factor Va is subsequently inactivated by further action of thrombin, thereby providing a means of limiting the activation of prothrombin to thrombin.
(iii) Formation of thrombin:
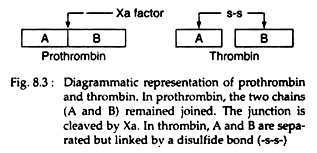
Prothrombin, a glycoprotein binds with the complex of factors Va and Xa (Fig. 8.2) on the platelet membrane. Factor Xa cleaves the prothrombin at two sites, to generate the active, two-chain thrombin molecule. This thrombin molecule is then released from the platelet surface. The A and B chains of the thrombin are held together by a disulfide bond (Fig. 8.3):
(iv) Role of Fibrinogen:
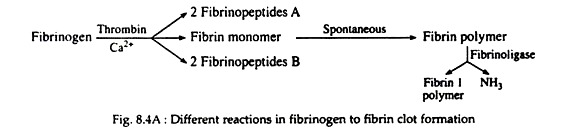
Fibrinogen (Fig. 8.4A) is a soluble plasma glycoprotein that consists of three non-identical pairs of polypeptide chains (Aα, Bβ, Ƴ)2 covalently linked by disulfide bonds. The A and B portions of the Aα and Bβ chains are designated as fibrinopeptides A (FPA) and fibrinopeptides B (FPB) respectively.
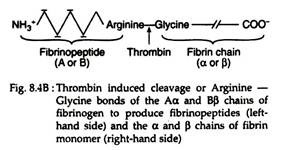
Thrombin hydrolyzes the bonds between the FPA and FPB and α or β portion (Fig. 8.4B) of the fibrinogen. The removal of fibrinopeptides generates fibrin monomer with the sub-unit structure (αβƳ)2. These monomers have exposed binding sites that allow the molecules of fibrin monomers to aggregate spontaneously in a regularly staggered array, thus forming an insoluble fibrin clot or fibrin polymer (Fig. 8.4A). This cross-linked fibrin polymer traps platelets, red cells and other components to form either the white or red thrombi.
Initially these polymers are weak, held together by non-covalent association of monomers. The strong and stable peptide bonds are formed between monomers by factor XIIIa (Fig. 8.2). The fibrin stabilising factor (factor XIII) is activated by thrombin to factor XIIIa. Factor XIIIa is a transglutaminase called fibrinoligase.
Mechanism # 4. Growth of Fibrous Tissues into the Blood Clot to Close the Hole:
The clot is composed of a meshwork of fibrin threads running in all directions and entrapping blood cells, platelets and plasma. The fibrin threads adhere to damage surfaces of blood vessels. Within a few minutes after a clot is formed, it begins to contract and usually squeeze out most of the fluid from the clot, within 30 to 60 minutes.
As the clot retracts, the edges of the broken blood vessel are pulled together, thus possibly or probably contributing to the ultimate state of haemostasis.
Role of Ca++ in the clotting mechanism:
Except for the first two steps in the intrinsic pathway, Ca++ ions are required for promotion of all of the reactions. Therefore, in the absence of calcium ions, blood clotting will not occur.