Contents:
- Modes of DNA Replication
- Conservative Replication and Dispersive Replication
- Ingredients Required for the Synthesis of DNA in E. coli (In Vitro)
- Molecular Basis of Replication in E. Coli
- Discontinuous Synthesis — Okazaki fragments, Leading Strand and Lagging Strands
- Removal of RNA Primers; Gap Filling; Proof Reading and Edition; Joining of Okazaki Fragments
1.
Modes of DNA Replication:
Replication is one of the essential properties of genetic material because the progeny cells should have the same genetic information as the parental cell. Once the structure of DNA was established by Watson and Crick, research was intensified to find out mechanism of DNA replication. Actually, the structure of the double helix itself suggests how replication could occur.
Modes of DNA Replication are:
ADVERTISEMENTS:
Semiconservative replication:
Watson and Crick proposed that if DNA double helix were unwound, each strand could serve as a template for the synthesis of a new complementary strand. Nucleotides along the two parent strands would have an affinity for its complementary nucleotides.
For example, if thymidylic acids (T) were present, it would attract adenylic acids (A); if guanidylic acids (G) were present, it would attract cytidylic acid (C) and so on. If the required nucleotides were then covalently linked into polypeptide chains along two templates, the result would be the production of two progeny DNA double helices, each consisting of one ‘old’ (parental) and one ‘new’ strand.
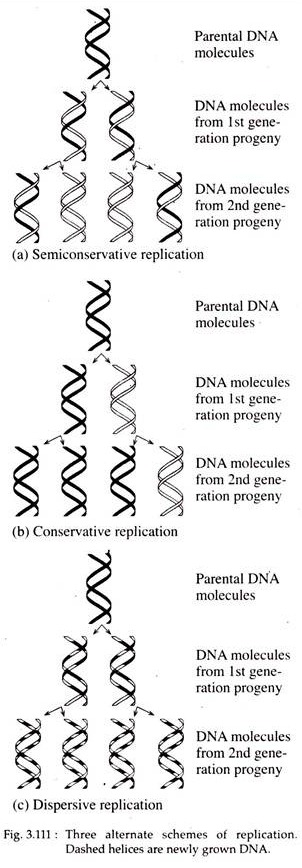
This type of replication is called semiconservative replication, since each progeny molecule retains one of the parental strands (Fig. 3.111a). In E. coli DNA replication occurs through this process.
ADVERTISEMENTS:
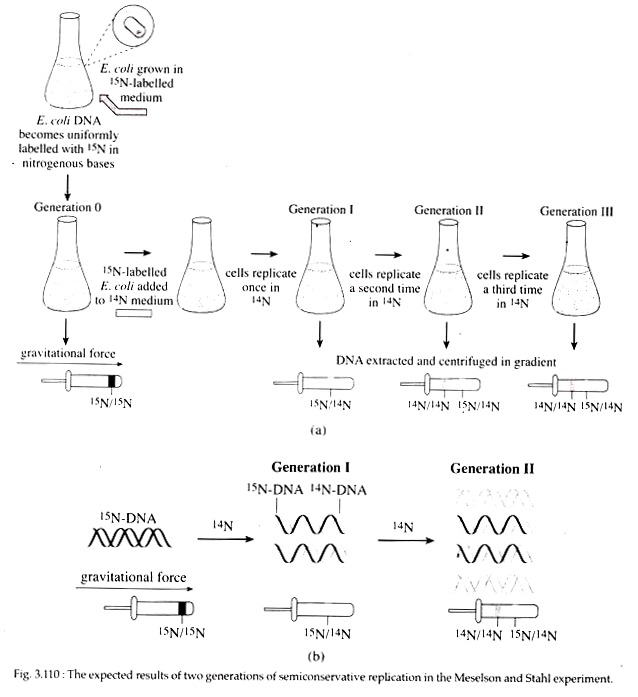
Meselson and Stahl Experiment:
In 1958, Mathew Meselson and Frank Stahl experimentally proved the semiconservative mode of replication in E. coli. They grew E. coli for several generations in a minimal medium in which 15NH4C1 (ammonium chloride) was the only source of nitrogen.
In this medium, normal nitrogen isotope 14N was replaced with heavy isotope 15N (density = weight/ volume; 15N with one extra neutron in its nucleus is 1/14 more dense than 14N). Following many generations of growth, all nitrogen containing molecules including the bases of DNA in the E. coli cells contained 15N.
These 15N labelled bacteria were then transferred to a medium containing only 14NH4C1 and allowed to reproduce in this medium for several generations. During this period of growth in the 14NH4C1 medium, samples of E. coli were removed at various intervals, lysed to release the cellular con tents and the DNA was analysed in cesium chloride density gradient (Fig. 3.110).
After one generation, the isolated DNA had a density that was exactly intermediate between that of totally 15N DNA and totally 14N DNA. After two generations, half the DNA was of the intermediate density and half was of the density of 14N DNA. This observation was consistent with the semiconservative replication where each replicated molecule after one generation consisted one new 14N strand and one old 15N strand.
2. Conservative Replication and Dispersive Replication:
According to conservative models two parental strands of DNA serve as templates for the synthesis of new progeny DNA double helices but the parental strands re-anneal after replication. Thus, one of the two daughter DNA molecules is actually the parental double-stranded DNA molecule and the other consists of totally new strands (Fig. 3.111b).
In the dispersive type, the parental double helix is cleaved into double stranded DNA segments which act as template for the synthesis of new double-stranded DNA segments. The segments then reassemble into complete DNA double helices consisting of both parental and progeny DNA segments interspersed.
Therefore, the two daughter DNAs are identical with respect to base pair sequence and the parental DNA become dispersed between both progeny molecules (Fig. 3.111c).
3. Ingredients Required for the Synthesis of DNA in E. coli (In Vitro):
In 1955, Arthur Kornberg and his colleagues first successfully synthesised E. coli DNA in vitro. They pointed out the necessity of the following ingredients for the synthesis of DNA in vitro.
(a) Four dNTPs e.g., dATP, dTTP, dGTP and dCTP are the precursor for the nucleotide building blocks of DNA.
ADVERTISEMENTS:
(b) Magnesium ions (Mg2+).
(c) A fragment of DNA — it acts as the primer; a primer is a pre-existing polynucleotide chain in DNA replication to which new nucleotide can be added.
(d) DNA polymerases: By definition, enzymes that catalyze the synthesis of DNA are called DNA polymerases. Kornberg isolated one DNA polymerase from the E. coli lysate that was capable of DNA synthesis. This enzyme was originally called the Kornberg enzyme, but now most commonly called DNA polymerase I (DNA pol I).
It consists of a single polypeptide chain and possesses three enzymatic activities:
1. It catalyses the formation of a phosphor-diester bond between the 3’OH group of
the deoxyribose on the last nucleotide and the 5’phosphate of the deoxyribonucleotide 5’triphosphate (dNTP) precursor. This formation of the phosphodiester bond results in the release of two of the three phosphates from the dNTP. In this reaction, the lengthening DNA chain acts as a primer.
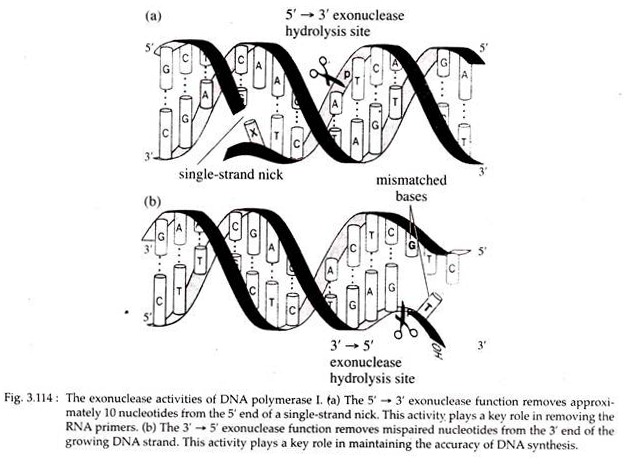
2. The enzyme has 5′ → 3′ exonuclease function, i.e., it cleaves a DNA strand from the free 5’P end.
3. Moreover, it has 3′ → 5′ exonuclease activity i.e., digests a DNA strand from free 3’OH end to 5’P end.
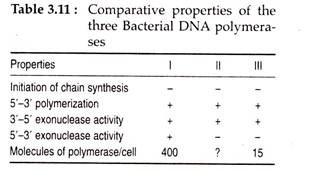
About 400 molecules of this enzyme are present in an E. coli cell. Due to this abundance it was held to be the true replicase. But is 1969 P. DeLucia and J. Cairns isolated polymerase A” mutants of E. coli which contained no active form of “Kornberg enzyme” but appeared capable of normal DNA replication.
Later on, two other E. coli polymerases; DNA polymerase II (DNA pol II) by M. Gefter, R. Knippers and C. C. Richardson (1970) and DNA polymerase III (DNA pol III) by T. Kornberg and M. Gefter (1971) were discovered.
These enzymes can check the accuracy of the recently assembled base pairs and if wrong base pair has been put together, DNA synthesis will stop. In such case, the enzymes show the 3′ → 5′ exonuclease activity and catalyze the excision of the erroneous nucleotide on the primer strand.
The polymerase enzymes then catalyze the formation of correct base pair. This process is called proof reading that keeps the frequency of DNA replication error very low.
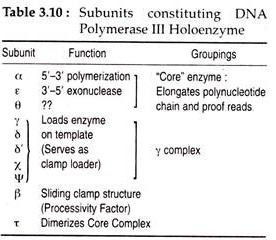
The active form of DNA pol III or the holoenzyme consists of two sets of 10 separate polypeptide units with different functions. During replication, the holpenzyme together with several other proteins form a large complex at the replication fork and is referred to as replisome. The functions of different subunits of DNA pol III and some of the important properties of three E. coli polymerases are given in Tables 3.10 and 3.11.
4. Molecular Basis of Replication in E. Coli:
The general mode of replication of DNA in E. coli is semiconservative.
However, the detailed pattern of replication with different relevant issues may be discussed as follows:
Initiation of Replication: Formation of Replication Bubble:
Replication of bacterial DNA usually starts at a specific point on the circular chromosome, the origin, where the double helix denatures into single strand, exposing the bases for the synthesis of new strands. Such local denatured part of the DNA double helix is called replication bubble.
The untwisted single strands, upon which the new strands are made, are called template strands. In the circular chromosome of E. coli, there is a single origin of replication, called ori C or replication origin and it remains anchored to the cell membrane from where replication proceeds.
The length of DNA that is replicated following one initiation event at a single origin is a unit and is referred to as replicon. The ori C has been mapped along the chromosome and it consists of 245 base pairs with repeated sequences of 9 and 13 bases called 9 mers and 13 mers.
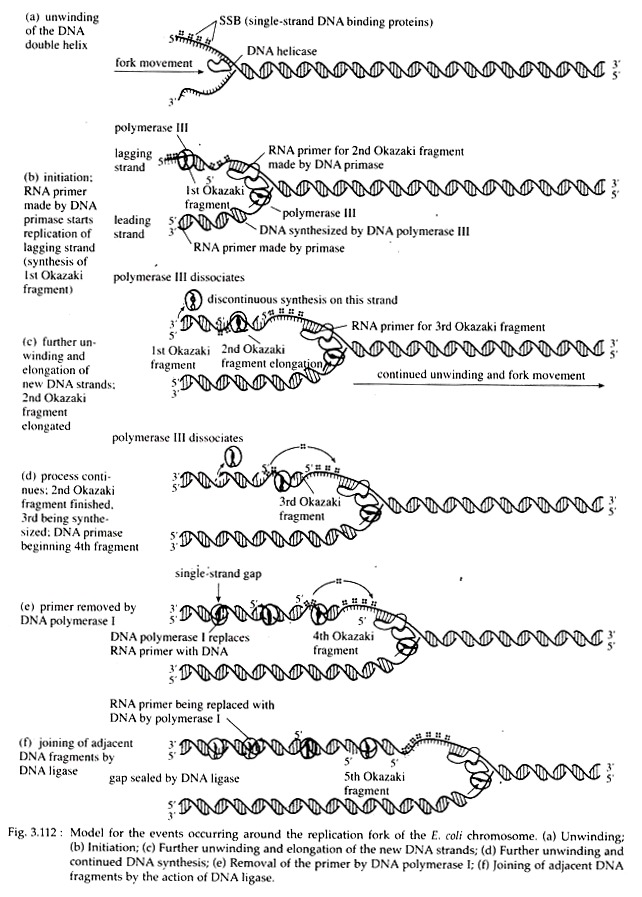
Since there is a single point of origin of DNA synthesis in bacteria, the entire circular chromosome constitute one replicon. During replication in E. coli, the circular DNA molecule exhibits a theta-like (0) shape because of the replicating bubble’s initiation at the replication origin (Fig. 3.112).
Unwinding of DNA duplex:
A parental DNA double helix should at first be unwounded so that the bases of the two template strands are available to the replication enzymes. In e. coli, a protein called Dna A (encoded by the gene Dna A) is responsible for the initial step in unwinding the helix.
A number of subunits of this protein bind to each of several 9 mers. This binding causes subsequent binding of Dna B and Dna C proteins that further open and destabilize the helix. The enzymes that break hydrogen bonds and denature the double helix, are called ‘helicase’, a ‘rep’ protein. Other proteins, called single-stranded binding proteins (SSBPs), stabilize this conformation.
Prevention of positive supercoiling:
As unwinding proceeds a coiling tension is created ahead of the replication fork, often producing supercoiling. Such supercoiling can be relaxed by the DNA gyrase, a member of a larger group of proteins referred to as DNA topoisomerases.
The gyrase makes either single- or double-stranded ‘cuts’ and causes ‘undoing’ of the twists and knots created during supercoiling. The strands are then released. All these reactions are driven by the energy released during ATP hydrolysis.
Formation of replication fork, RNA primer:
When a double-stranded DNA molecule unwinds to expose the two single- stranded template strands for DNA replication, a Y-shaped structure called a replication fork is formed. No DNA polymerases can initiate the synthesis of a DNA strand; they can only catalyze the addition of deoxyribonucleotides to a pre-existing strand.
Following activation by helicase, the enzyme DNA primase (product of Dna G gene) synthesizes a short segment of RNA (5 to 15 nucleotides long), complementary to DNA on the template strand. The primer starts with two purine nucleotides, most frequently AG. The complex of the primase and the helicase with the DNA is called the primo-some (Fig. 3.112).
Chain elongation:
Following the synthesis of primer, DNA pol III first recognizes a base on the DNA template and mediates the incorporation of an exactly complementary nucleoside triphosphate, opposite to it.
The new nucleotides for the daughter strand are supplied from four types of nucleoside triphosphates like dATP, dCTP, dGTP and dTTP, instead of four types of nucleoside monophosphates as such. Moreover, for in vitro DNA synthesis, all four types of nucleoside triphosphates are to be supplied in 1 :1 : 1 : 1 proportions. This is called limited reaction.
The enzyme then catalyzes the formation of 3′, 5′-phosphodiester bond between the first or a-phosphate of the incorporated nucleoside triphosphate and the free 3’OH of the primer. At this time a molecule of pyrophosphate (P~P) is released from the 2- terminal phosphates of the used nucleoside triphosphate.
Pyrophosphate when hydrolyzed into 2 phosphates, yields energy which drives the elongation process forward. In this way chain elongation continues. At a later point, the RNA primer is removed hydrolytically by 5′ → 3’exonuclease action of DNA Pol I.
5. Discontinuous Synthesis — Okazaki fragments, Leading Strand and Lagging Strands:
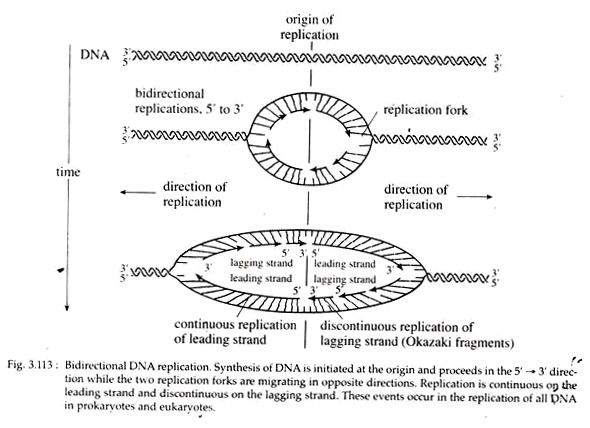
DNA polymerases can elongate chains only from the 3’OH end of the template. But there are two templates in one DNA molecule. So it is difficult to get both strands replicated at the same time since the two strands have opposite polarity i.e., one strand’s 3’OH is opposed to 5’P of the other.
As the replication fork progresses down the helix, synthesis of the new strand occurs easily and possibly continuously on the template strand. This daughter strand is called leading strand. Synthesis of the other new strand must be discontinuous because the helix must untwist to expose a new segment of 3′ → 5′ template, so that the new strand can be made in the 5′ → 3′ direction.
A short primer is then synthesized near the forked region along the other template and ahead of this primer, new strand is synthesized in the form of short piece. When further unwinding of parental duplex occurs, another small piece is synthesized behind this small piece, and ahead of another RNA primer piece.
This new strand consisting of many small, discontinuous pieces is called lagging strand. These small pieces of lagging strand containing 1,000 to 2,000 nucleotides are called Okazaki fragments after the discoverer, R. Okazaki. Since one new strand is synthesized continuously and the other discontinuously, DNA replication as a whole is considered to occur in a semi-discontinuous fashion (Fig. 3.113).
Pulse-chase Experiment:
Evidence in support of semi-discontinuous DNA replication was first provided by Reiji Okazaki, Tuneko Okazaki and their colleagues. In their experiment, cells were exposed, for a brief period to 3H-thymidine. Following isolation of their DNA, most of the radioactivity is found in low molecular weight DNA strands which consists of about 1,000 nucleotides.
After short pulse of 3H-thymidine, the cells were washed, given un-labelled thymidine and allowed to replicate for an additional short period. The label was then found in high molecular weight DNA pieces indicating that Okazaki fragments were subsequently joined to form a continuous daughter strand.
During replication, synthesis of new strands along an advancing replication fork occurs in one direction on one strand and in the opposite direction on the other one, simultaneously. This is called bidirectional replication (Fig. 3.113).
6. Removal of RNA Primers; Gap Filling; Proof Reading and Edition; Joining of Okazaki Fragments:
DNA pol I digests the RNA primers from the ends of Okazaki pieces by means of its 5′ → 3’exonuclease activity. The gaps created following the removal of RNA primers are possibly filled in by new deoxyribonucleotides with the help of polymerase activity of DNA pol I.
After removal of RNA primers, the Okazaki fragments are joined by DNA ligase which in E. coli is specified by lig-gene. Mutants defective in ligase activity join Okazaki pieces very slowly. Then by gyrase action, the template and daughter strands form right handed coils around each other and thus daughter DNA formation is completed.
Occasionally error occurs in DNA replication when incorrect nucleotide is incorporated into the strand that is being synthesized. Somehow the mismatched base pair at the growing 3′ end of the new strand triggers the 3′ → 5′ exonuclease activity of DNA polymerases which then back up one base on the template strand and removes the incorrect base on the new strand (Fig. 3.114).
The polymerases then resume the forward direction and continue nucleotide addition.
Both DNA pol I and III can detect and excise such mismatched nucleotides by the process of proof reading and correction, respectively. Recognition of wrong base incorporation is called proof reading and correction is called edition.
So, it appears that DNA replication in E. coli is a complicated process and it successfully continues because of the coordinated action of different proteins, which are closely associated to form a replication machine or replisome.
This replisome moves as a unit along the DNA and enables new DNA to be synthesized efficiently on both the leading strand and lagging strand templates. Mg++ acts as a cofactor for DNA polymerase enzymes, and ATP and GTP supply energy.