Zoology Notes on Genetic Recombination:- 1. Meaning of Genetic Recombination 2. Molecular basis of Genetic Recombination 3. Gene Conversion and Crossing-over.
Meaning of Genetic Recombination:
The term recombination is widely used in different fields of genetics. Literally recombination means the process of genetic exchange between the parental chromosomes during meiosis in diploid organisms where the parental chromosomes are “cut up and pasted together”.
Morgan and his colleague E. Cattell first introduced the term ‘crossing- over’ to describe the process of exchange of material between homologous chromosomes by which new combinations of linked genes or recombinants arise.
The actual process of crossing-over is the reciprocal exchange of chromatid segments at corresponding position along homologous chromosomes; it involve symmetrical breakage of two non-sister chromatids and their rejoining. The place on the homologous pair of chromosomes at which physical exchange takes place is referred to as ‘chiasmata’.
ADVERTISEMENTS:
Crossing-over takes place at late pachytene stage of first prophase of meiosis. It results in reshuffling or recombination of the alleles between homologues. However, physical exchange between chromosomes leading to genetic recombination was just a hypothesis, at the time of Morgan.
The first evidence for the association of gene recombination with chromosomal exchange came in 1931, following the work of Harriet B. Creighton and Barbara McClintock with corn (Zea mays).
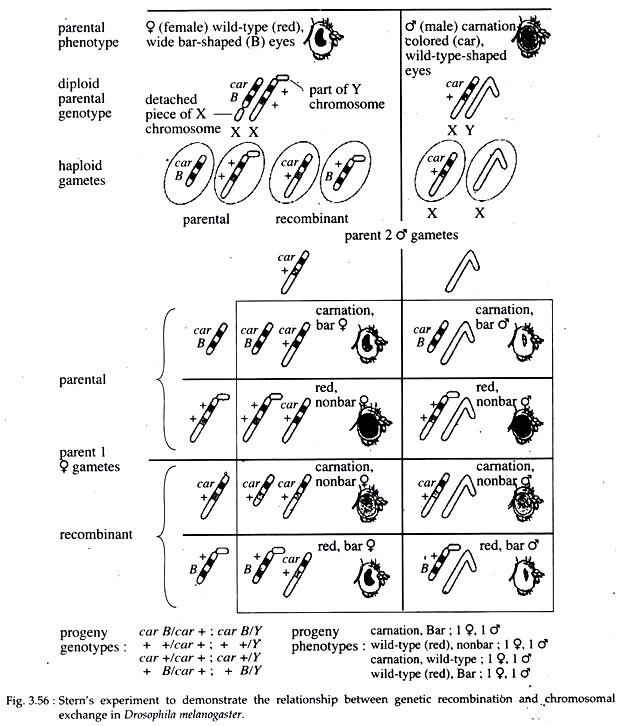
They found that whenever genes had recombined, the cytological features had also recombined. In the same year, Curt Stern reported identical conclusions for experiment done with Drosophila. He selected two linked gene loci; the car for carnation coloured eye gene and the B (for bar shaped eye) gene.
In the crosses, the male parent carried normal X and Y chromosomes with the recessive allele car and wild-type allele of the B gene. Since males are hemizygous, they had non bar, carnation-coloured eyes.
The females had two abnormal and cytologically distinct X chromosomes. One X-chromosome carried the wild type alleles of both the car and the B genes and it had a portion of the Y- chromosome attached to it. The other X-chromosome had the recessive car allele and the dominant B allele.
Moreover, the latter X- chromosome was distinctly shorter than normal X since part of it had broken off and was attached to the small chromosome 4 (Fig. 3.56). Phenotypically, this parental females had a wide-bar eye since they were B / +, and the eye was brick red (wild-type) in colour, since they were heterozygous +/ car.
During gamete formation, two classes of sperm were produced: the Y-bearing and the X-bearing with car and B+ alleles. However, four classes of eggs were produced. Two were parental type in which no crossing-over had occurred, and the other two were recombinant gametes produced by crossing-over.
If no recombination occurred, the two phenotypic classes of progeny were:
ADVERTISEMENTS:
(a) Red-eyed with normal eye shape; and
(b) Carnation-coloured eye with bar shape.
There also were two classes of recombinant:
(i) Carnation coloured with round eyes; and
(ii) Red coloured with bar shaped eye.
The carnation flies had a complete X-chromosome, and the bar flies had a shorter than normal X-chromosome to which a piece of Y-chromosome was attached, while the rest of the X-chromosome was attached to chromosome 4.
These chromosomal combinations could only have been produced from physical exchange of homologous chromosomal part. So, it firmly establishes that ‘genetic recombination’ results from physical crossing-over between chromosomes.
Molecular basis of Genetic Recombination:
After the elaboration of DNA structure by Watson and Crick, several scientific models were put forward to explain the molecular mechanism of crossing-over. Robin Holliday, in 1964, described the first model.
Thereafter substantial evidences suggested that mechanism for crossing-over may not be the same among all species and thus Holliday model may not be entirely correct. Nonetheless, the Holliday model does explain a number of observations including gene conversion and is therefore accepted till now.
ADVERTISEMENTS:
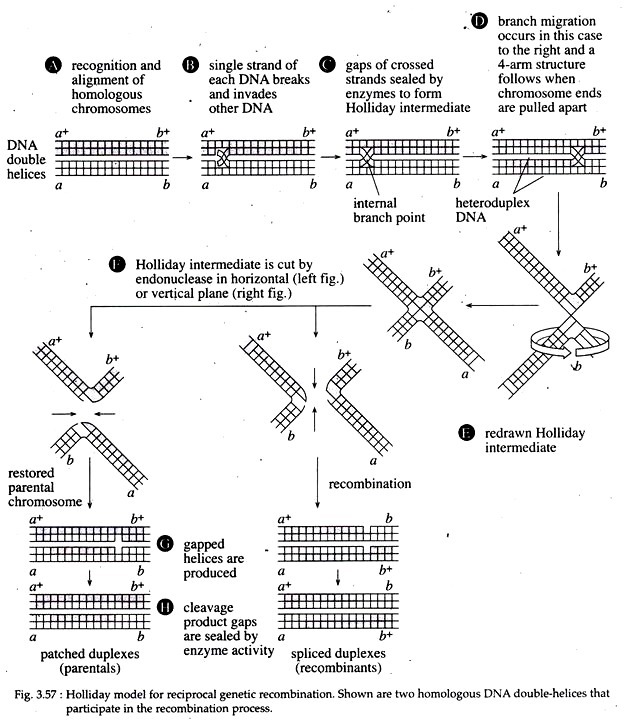
According to this model, the first stage of recombination process is recognition and alignment (Fig. 3.57A). During this stage, two homologous DNA double helices become precisely aligned so that no genetic material is added or deleted to either DNA in subsequent stages. In eukaryotes, this process is called synapsis, which is accompanied by the formation of a synaptonemal complex.
In the second stage, an endonuclease introduces a ‘nick’ at an identical position of one strand of each double helix. Each broken strand then invades the opposite double helix (Fig. 3.57B). The gaps created in this process are sealed by DNA polymerase and DNA ligase. The hybrid DNA molecules evident at this stage are called heteroduplex DNA molecules.
The two strands of the double-stranded DNA molecules do not have complete complementary sequences. At the internal branch point, also called Holliday intermediate (Fig. 3.57C), the two DNA double helices can rotate, causing the branch point to move to the right or to the left by a process referred to as branch migration.
This occurs as a result of a zipper-like action as hydrogen bonds are broken and then reformed between complementary bases of the displaced strands of each duplex. This migration yields an increased length of heteroduplex DNA on both homologues that may well contain mismatched base pairs; the mismatches are subject to repair.
Now, if the lower two arms of the four armed Holliday intermediate (as shown in Fig. 3.57D) separate and rotate 180° relative to the upper arms, an intermediate planer structure called a ‘chi’ is created (Fig. 3.57E). Next, the endonuclease cuts the Holliday intermediate at two points in the single stranded DNA region of the branch point.
The cuts can be in either horizontal or vertical planes. If the endonuclease cut is horizontal, in each helix, there occurs a single-stranded gap that is sealed by DNA ligase to produce the double helices. Each of the resulting helices contains a segment of single stranded DNA from the other helix, flanked by non-recombinant DNA. These double helices thus are called patched duplexes (Fig. 3.57G, H left hand figures).
If the endonuclease cut is vertical, there are hybrid DNA in each duplex that are formed by what looks like a splicing together of two helices. The resulting double helices are called spliced duplexes (Fig. 3.57G, H right hand figures).
In the given figure (Fig. 3.57G, H), the parental duplexes contain different genetic markers at the end of the molecule. One parent has a+b+ and the other has ab as would be the case for a doubly heterozygous parent.
However, following reciprocal recombination, different products are produced. In the patched duplexes, the markers are a+b+ and ab, same as that of parental configuration (Fig. 3.57A, H). But in the spliced duplexes, the markers are recombinant, a+b and ab+ (Fig. 3.57H right hand figure).
Since enzymatic cut during cleavage and ligation phase occur randomly, the Holliday model predicts that a physical exchange between two gene loci on homologous chromosomes results in the genetic exchange of the outside chromosome markers, by about half of the time.
It thus appears that crossing-over is a precise process during which no chromosome segments are lost or gained and four complete chromosomes emerge in a tetrad. Several modifications of the Holliday model provide interesting information.
However, the actual mechanism of crossing-over is still somewhat of an enigma. In 1975, Matthew Meselson and Charles Radding proposed the asymmetric strand- transfer model, that differs from the Holliday model in that it proposes a transfer of only one DNA strand (instead of two).
According to this model, a heteroduplex can be present on only one molecule if there is no branch migration. If branch migration occurs, heteroduplex are present in both molecules but not equally. But, in the Holliday model, heteroduplex strands are equally present in both molecules for every gene conversion.
Another modification is the double- strand-break repair model. According to this model, a double-strand break in one molecule creates two Holliday junctions and four possible outcomes – two causes a cross-over and two do not. However, all such outcome may result from gene conversion.
Unlike the Holliday model, it proposes that heteroduplex regions are located at different positions in the two DNA molecules. However, the double-strand- break repair model may be the mechanism of recombination in yeast and also in other eukaryotes.
However, although different models differ in the explanation regarding the mechanism of crossing-over, each model relies on the role of different enzymes that accomplish genetic recombination. For example, the rec A protein, discovered from E. coli promotes the exchange of reciprocal single- stranded DNA molecules.
It also enhances the hydrogen bond formation during strand displacement and thus helps in heteroduplex formation. Other enzymes produced from rec B, rec C and rec D genes are thought to be involved in the nicking and unwinding of DNA.
Gene Conversion and Crossing-over:
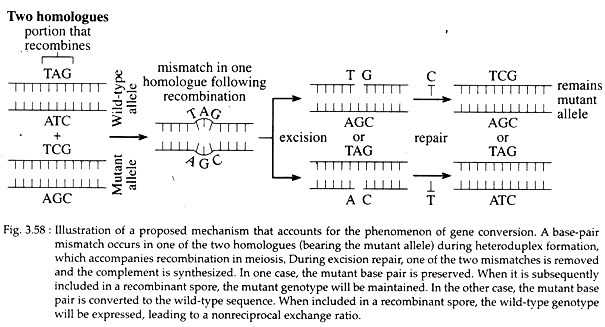
Gene conversion is the process of non-reciprocal genetic exchange between two closely linked genes. It can occur only where there is heterozygosity for two different alleles of a gene.
It could be explained as a mutation that changes an allele a to A or A to a, but it is much more frequent than mutation and about 50% of all gene conversions are associated with a cross-over, suggesting that gene conversion is somewhat related to crossing-over.
Initially it was found in yeast by Carl Lindegren and in Neurospora by Mary Mitchell. Mitchell crossed two strains of Neurospora, one strain has two genes a + and the other has mutant genes + b. Reciprocal recombination (a + X + b) between the genes would yield spore pairs of the ++ and the ab genotypes.
Whereas, non-reciprocal exchange, by contrast, would yield one pair without the other. While working with pyridoxine (a nutritional substance) mutants, Mitchell observed several asci containing spore pairs with the 4-4- genotype, but not the reciprocal product (ab).
Mitchell found that the frequency of these events were higher than the rate of mutation and so could not be accounted for by mutation. (Moreover, the process is directional the allele that is converted always changes to the other specific allele involved in the cross).
It appeared that one allele had somehow been ‘converted’ to another where genetic exchange had also occurred. So it is now considered to be a consequence of DNA recombination.
Gene conversion is also explained as the mismatch of base pairs during heteroduplex formation, (Fig. 3.58) that can be repaired by the excision of one of the strands and the synthesis of the complement using remaining strand as a template. Conversion may have the effect of creating identical alleles on the two homologues that were different initially.