The below mentioned article provides an overview on the Inheritance of Autosomal and Sex-linked Genes in Man:- 1. Introduction to Inheritance of Autosomal 2. Inheritance of Autosomal Recessive Genes 3. Inheritance of Autosomal Dominant Genes 4. Inheritance of X-linked Recessive Genes 5. Inheritance of X-linked Dominant Genes and Other Details.
Contents:
- Introduction to Inheritance of Autosomal
- Inheritance of Autosomal Recessive Genes
- Inheritance of Autosomal Dominant Genes
- Inheritance of X-linked Recessive Genes
- Inheritance of X-linked Dominant Genes
- Inheritance of Y-linked Genes
- Haemophilia: X-linked Recessive Trait
- Colour-blindness: X-linked Recessive Trait
- Thalassemia’s – Autosomal Recessive Trait
1. Introduction to Inheritance of Autosomal:
After the rediscovery of Mendel’s laws in 1900, geneticists found that Mendelian principles of gene segregation are applicable to all eukaryotes, including humans. In 1902 Walter Sutton and Theodar Boveri independently recognized that the transmission of chromosomes from one generation to the next, closely paralleled the pattern of transmission of genes from one generation to the next.
ADVERTISEMENTS:
To explain this correlation they proposed the chromosome theory of inheritance which states that chromosomes are the carriers of genes.
The study of the inheritance of genetic traits in humans is complicated by the fact that no controlled crosses can be made. Instead, human geneticists analyzed genetic traits by pedigree analysis, that is, by examining the occurrences of the trait in family trees of individuals, who clearly exhibit that trait.
In human, out of 46 chromosomes, 44 are autosomes and rest two are sex chromosomes. Genes responsible for a trait may lie either on autosomes or on sex chromosomes. Since, in a diploid cell, there are two of each kind of autosome, the autosomal genes are present in double doses. The inheritance pattern of genes lying on the autosomes is called autosomal inheritance.
On the other hand, human females have two X chromosomes and males have one X and one Y chromosome as their sex chromosome. During meiosis, if X and Y chromosomes are to synapse and segregate, the Y chromosome must contain a region of pairing homology with the X-chromosome.
ADVERTISEMENTS:
On the human X and Y chromosomes, two such areas have been identified, one on each end of both chromosomes, referred to as pseudo-autosomal regions (PARs). However, the remainder part of the Y chromosome in human lack homology with almost all genes present on the X chromosome.
As a result genes present on the X chromosome exhibit unique pattern of inheritance in comparison to autosomal genes that is called X-linked inheritance or X-linkage. On the other hand, most of the alleles that are present on X chromosome of the male will be directly expressed in the phenotype of male.
So, males cannot be either homozygous or heterozygous for X-linked genes; this condition is referred to as hemizygous. (Modern molecular techniques have discovered a few human Y-linked genes that have counterparts on the X-chromosome).
However, phenotypic expression is not always the direct reflection of the genotype and it may be modified by genetic background, temperature and nutrition. Genes either autosomal or sex-linked, dominant or recessive are inherited following certain rules.
ADVERTISEMENTS:
ADVERTISEMENTS:
2. Inheritance of Autosomal Recessive Genes:
A large number of human traits are known to be caused by homozygosity for mutant alleles that are recessive to the normal allele. Such recessive mutant alleles produce mutant phenotypes because of a loss of function of the gene product resulting from the mutation involved.
Many serious abnormalities or diseases result from homozygosity for recessive mutant alleles like Albinism, Bloom syndrome, Phenylketonuria, Thalassemia and Falconi Anaemia, etc.
The inheritance pattern of these traits shows some general characteristics as follows:
i. Most affected individuals have two normal parents, both of whom are heterozygous. The trait appears in the F, since a quarter of the progeny are expected to be homozygous for the recessive allele.
ii. Mating between two normal heterozygotes should produce an approximately 3 : 1 ratio of normal progeny to progeny exhibiting the recessive trait.
iii. Mating between one normal heterozygote and one affected homozygote will produce half affected and half normal heterozygote offspring’s.
iv. When both parents are affected, all their progeny will usually exhibit the trait.
3. Inheritance of Autosomal Dominant Genes:
ADVERTISEMENTS:
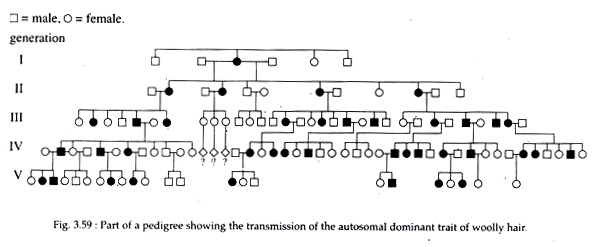
The best example of autosomal dominant trait in human is woolly hair (Fig. 3.59). Other includes Brachydactylic, Nail Patella syndrome, Huntington’s disease, Hypercalcemia, Marfan syndrome, etc.
Dominant mutant alleles are expressed in a heterozygote when they are in combination with what is called usually the wild-type allele — the allele that predominates in population found in the ‘wild’. In human, however, abnormalities caused by dominant, mutant alleles generally expressed at older age e.g., Huntington’s disease.
General characteristics of a dominant trait are as follows:
i. Every affected person in the pedigree must have at least one affected parent.
ii. The trait usually will not skip generations.
iii. An affected heterozygous individual will, on average, transmit the mutant gene to half of his or her progeny.
4. Inheritance of X-linked Recessive Genes:
A trait due to a recessive mutant allele carried on the X-chromosome is called an X- linked recessive trait. At least 100 human traits are known for which genes have been traced on X-chromosome. Such genes show crisscross pattern of inheritance, whereby the recessive X-linked genes are passed from homozygous mother to all her sons.
The instance of a father-to-son inheritance is rare. Human X-linked recessive traits include Colour blindness (both deutan and protan type), Fabryt’s disease, G-6-PD deficiency, Hemophilia A and B, Muscular dystrophy, Ichthyoids, Hunter syndrome, etc.
Characteristics of X-linked recessive inheritances are as follows:
i. More males than females exhibit the trait, owing to different number of X-chromosomes in the two sexes.
ii. All sons of a homozygous mutant mother should show the trait. The sons of heterozygous mothers should show an approximately 1 : 1 ratio of normal individuals, to individuals expressing the trait.
iii. Mating between an affected male and pure normal female will produce all normal (apparent) children.
5. Inheritance of X-linked Dominant Genes:
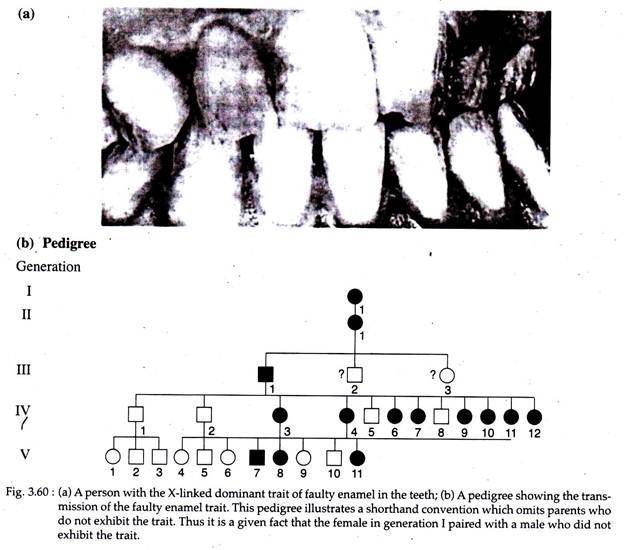
Only a few X-linked dominant traits have been identified in human, like Faulty tooth enamel (Fig. 3.60), Webbing at the tips of the toes and Constitutional thrombopathy.
The X-linked dominant traits follow the same mode of inheritance as that of X-linked recessive trait, but in case of the former, heterozygous females express the trait. Since females have twice the number of X-chromosomes as males, X-linked dominant traits are more frequent in females than in males.
Heterozygous females will pass the trait on to half of their sons and to half of their daughters. On the other hand, a male with an X-linked dominant trait will pass the trait on to all of his daughters and to none of his sons.
6. Inheritance of Y-linked Genes:
A trait due to a mutant gene carried on the Y-chromosome but with no counterpart on the X is called a Y-linked, or holandric (wholly male) trait. Such traits should be readily recognizable, since every son of an affected male should have the trait and no females should ever express it. Possible examples of Y-linked inheritance are Hairy pinnae and Bilateral radio-ulna synostosis.
7. Haemophilia: X-linked Recessive Trait:
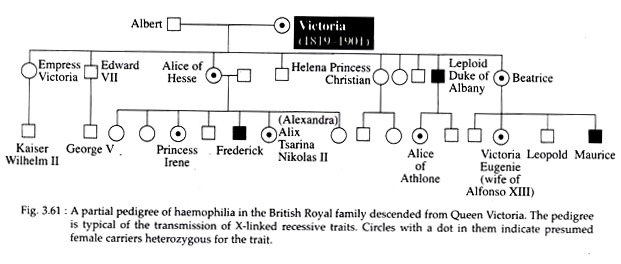
The best known X-linked recessive trait in human is haemophilia. It is a serious ailment in which the blood lacks a clotting factor, so a cut or even a serious bruise can be fatal due to haemorrage. In Queen Victoria’s pedigree, the first instance of haemophilia was found in one of her sons.
Since Queen Victoria was normal and didn’t express the symptoms of the disease, she was heterozygous for the sex-linked recessive haemophilia allele. No cases of haemophilia were reported in her ancestors, so it is thought that the trait must have arisen as a mutation in one of her parental germ cells (Fig. 3.61).
Types of Haemophilia:
In general, three types of haemophilia are known, each affecting the production of a prothrombin proteinase (factor X), an enzyme necessary for blood clotting.
Haemophilia A:
This ‘classic’ sex linked type of haemophilia accounts for approximately 80% of known haemophilia. In this disease, the factor VIII or antihaemophilic factor (AHF) is not present in the blood of the patients.
Haemophilia B:
This is also a sex linked type and characterised by the reduction in the amount of plasma thromboplastin component (PTC) or factor IX in the blood. It accounts for about .20% of haemophilias. The gene for haemophilia B is not allelic to that of haemophilia A.
Haemophilia C:
This disease is caused by a rare autosomal gene (here the gene is symbolized by a and the normal allele is a+) that interferes with the production of plasma thromboplastin antecedent (PTA) or factor XI, and is responsible for less than 1% of haemophilias.
Inheritance of Haemophilia:
The characteristics of inheritance of haemophilia are as follows:
i. More males than females exhibit the trait, owing to different number of X chromosomes in the two sexes (one in a male and two in a female).
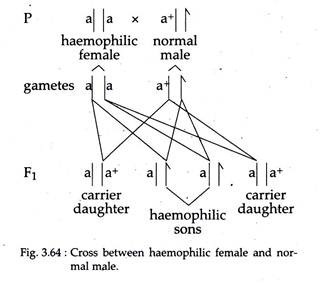
ii. All sons of a haemophilic (homozygous mutant) mother should show the trait, since males receive their only X-chromosome from their mother (Fig. 3.64).
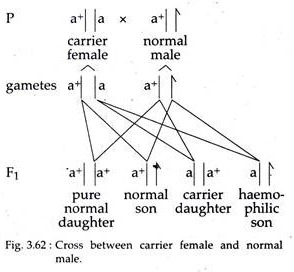
iii. The sons of an apparently normal mother (heterozygous carrier) should show an approximately 1 : 1 ratio of normal individuals to haemophilic individuals (Fig. 3.62).
iv. Marriage between a carrier (heterozygous) haemophilic female and a normal male will produce all apparent normal daughters but half will be carriers. In turn, half the sons of these carrier females will be haemophilic (Fig. 3.62).
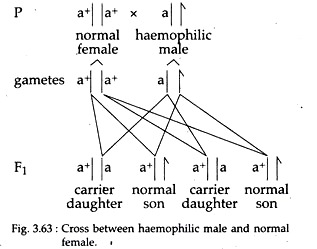
v. Marriage between a haemophilic male and a pure normal (homozygous) female will give rise to all apparently normal children; but all the female progeny will be carrier for haemophilia (Fig. 3.63).
8. Colour-blindness: X-linked Recessive Trait:
Colour blindness is a common abnormality due to a recessive mutation in human, in which affected persons cannot perceive either red or green colour. It is of two types. In deutan type, affected persons are insensitive to green light while in protan type insensitivity is found to red light.
Like several other types of recessive genetic diseases, colour blindness is also determined by genes located on the X- chromosome. However, unlike the sex linked fatal disease haemophilia, colour blindness has no observable viability effect. But the inheritance pattern is same as that of the haemophilia gene.
The X-chromosomes may have the two alternative allele such as Xc and Xc, the normal and colour-blind alleles, respectively. So in females, there are three different phenotypes (XCXC, XCXc, and XCXC) in which only the last is colour blind because the normal allele is dominant in heterozygotes.
As the Y chromosome does not carry the colour, blindness gene, the two types of male are XCY and XcY — normal and colorblind, respectively. In females, segregation of X-linked genes is the same as that of autosomal genes, while in males, half of the gametes carry X and half carry Y-chromosome.
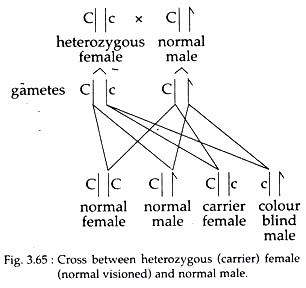
So cross between a woman heterozygous for colour blindness and a man with normal sight will produce different offspring’s as follows:
So, the ratio of normal to affected progeny is still 3 :1 but unlike the F2 progeny of mono-hybrid cross for an autosomal gene, all the recessive types are males. Pedigrees for X linked colour blindness traits are very similar as shown in pedigrees of haemophilia.
9. Thalassemia’s – Autosomal Recessive Trait:
Thalassemia’s are not a single disease, but a group of genetic disorders, involving chromosome 11 or 16 each of which results from an inherited abnormality in the production of mRNA for one of the two major adult haemoglobin protein, α-globin or β-globin.
Codey and Lee in 1925, first discovered a severe anaemia associated with splenomegaly and changes in bone, occurring early in life. This condition is later named as thalassemia. Incidences of thalassemia’s are quite frequent in Mediterranean region, central and equatorial Africa, north Europe, south Asia, Philippines and India. In India, thalassemia’s are more common among Sindhis and Kutchi-Lonanas.
The thalassemia’s are characterized by either reduced or total stoppage of synthesis of the affected globin chain. So a compensatory rise in the synthesis of other globin chains replace the missing globin chains in the haemoglobin molecules resulting in consequent formation of abnormal haemoglobin, premature destruction of erythrocytes, anaemia, bone marrow expansion etc.
Other symptoms include enlarged liver and spleen, thin long bones with hyperplastic bone marrow, retarded body growth etc.
Brief Structure of Human Haemoglobin:
As thalassemia’s are related to malfunction in globin production, a brief knowledge of the structure of haemoglobin is essential. Human adult haemoglobin (Hb) is a chromo- protein consisting of an apoprotein called globin, and a prosthetic group of Fe2+ protoporphyrin III (IX) compound called haeme.
The globin is a tetrameric basic protein of histone class. It is made of paired peptides of two different chains α and β. The a-like globin chains include the α-chains of foetal and adult haemoglobins and the δ chains of embryonic haemoglobin. The β-like globin chains include the β and δ chains of adult haemoglobin, γ chains of foetal and embryonic haemoglobin as well as the ε chains of embryonic haemoglobin.
Each of four pep- tide chains remain bound to a haeme molecule. About 97% of adult human haemoglobin consists of haemoglobin A (HbA). It’s globin consists of two a and two (3 chains (α2β2). About 2% of adult haemoglobin consists of haemoglobin A2 (HbA2) having α2β2 as its quaternary structure.
Foetal haemoglobin (HbF) has globin with a2y2 quaternary structure. HbA appears in the foetal blood by the 20th week of foetal life and progressively increases to 20% and 90% of the total haemoglobin at birth and four months after birth, respectively. About 1% of the haemoglobin in the adult human still consists of HbF (α2γ2).
Globin Genes:
In human, β-like globin genes compose a gene family closely linked together in a gene cluster on chromosome 11 in order ε—γ—δ—β the same order as their appearance during development. The cx-like globin genes are also a gene family; but located on chromosome 16, linked in order δ-α.
The exact mechanisms that regulate globin gene function are not yet clear. However, different types of mutation or change in these two gene clusters result in different types of thalassemia’s which may be discussed as follows.
Types of Thalassemia’s:
The alpha (α) and beta (β) thalassemia’s are caused by the mutations affecting the synthesis of globin chains. The affected globin chain is poor in amount in α+ and β+ thalassemia’s, but absent in α° and β° thalassemia’s.
α-thalassaemia:
It results from a reduced rate of production of a-chain. This defective synthesis in turn resulted from mutations in the a-gene cluster, more commonly from deletions and unequal crossing overs, and less commonly from frame shift mutations, nonsense mutations, and mis-sense point mutations.
For instance, in the last case, a Leu(Leucine) codon of α-2 gene is changed to a Pra (Proline) codon resulting in the abnormal Hb Quong Sze.
Again in one type of α-thalassemia, the stop codon UAA at the 142nd position in the α-globin mRNA has been changed into AAA encoding lysine; this elongate the a-globin chain from its normal 141-residue length to 172 – residues. All such abnormal α-globin chains occur in the abnormal Hb molecules of the patients with a-thalassemia’s.
α°-thalassemia’s:
It includes:
(i) The asymptomatic silent-carrier trait or α-thal-1 where a single a gene is deleted,
(ii) Mildly anaemic α-thalassemia trait or α-thal-2 with two missing α genes,
(iii) Moderately anaemic HbH disease with three deleted a genes and carrying significant amounts of abnormal homotetrameric HbH (β4), and
(iv) Severe and early lethal hydrops foetalis where all four a genes are deleted and embryonic δ globin chains are present in place of α chains in the abnormal haemoglobin.
β-thalassaemia:
It is caused by defective production of β chains. Mutations in the β- gene cluster, such as deletions of β genes causes β°-thalassaemias.
Other changes include:
(i) Point mutations of transcription- initiating promotor sites of β-genes affecting P-gene transcription,
(ii) Point mutations affecting an existing splice site or creating a new splice site to alter RNA processing and
(iii) Frame-shift and nonsense mutations of β-genes.
In all β-thalassaemias, the rate of β- chain translation is reduced which results in compensatory rise in the synthesis of δ and γ chains, and β-deficient haemoglobins such as HbA2 (α2δ2) and HbF (α2γ2) rise in blood, where normal HbA2 (α2δ2) is poor or absent. In one β°-thalassaemia, a stop mutation changes the 17th codon AAG (lysine) in the β-globin mRNA to the stop codon UAG.
This premature termination of translation causes absence of β-globin in the blood of the patients.
In δβ-thalassaemia patients with both δ and β genes from the β-like gene cluster are deleted, so a compensatory overproduction of foetal γ chain may produce sufficient amount of HbF (α2γ2) to make the patients asymptomatic.
In Hb C-β-thalassaemia, erythrocytes contain HbC made of two normal a chains and two abnormal β chains, each of the latter having Lys (Lysine) at position 6 of its peptide chain in place of Glu6 (Glutathione) of normal β chains — due to a transition type of point mutation.
Thalassaemia Major and Minor:
The thalassaemias are caused by recessive autosome gene. A homozygous individual, inheriting the mutant thalassaemic gene from both parents suffer from severe and often fatal anaemia and show other symptoms. This condition is referred to as thalassaemia major. These patients are unable to make β-chains and continue to grow α2γ2 globin chains even in adult condition and may need frequent blood transfusion.
A heterozygous individual, inheriting the thalassaemic gene from only one parent is either asymptomatic and apparently normal or only mildly anaemic. This condition is referred to as thalassaemia minor. Heterozygotic thalassaemic minor patients show higher resistance against the malarial parasite, Plasmodium falciparum.
Inheritance of Thalassaemias:
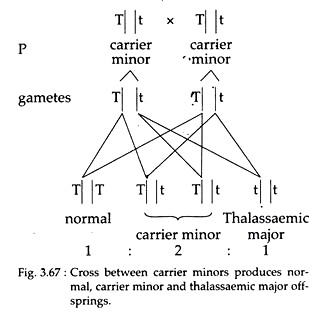
Homozygous individuals having both the recessive alleles of thalassaemia gene show the symptoms of thalassaemia major. On the other hand, heterozygous individuals having only one recessive allele are thalassaemic minor and are carrier.
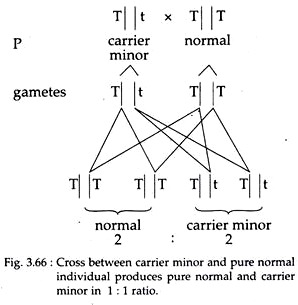
So marriage between one pure normal and one carrier minor individual will produce all apparent normal individuals of which pure normal and asymptomatic carrier will be in 1 : 1 ratio (Fig. 3.66).
Again, marriage between two carrier individuals will produce pure normal, carrier minor and major thalassaemic individuals in the ratio of 1 : 2 : 1, respectively (Fig. 3.67).