Contents:
- Introduction to Origin of Life
- Definition of Life
- Ancestral Home of life – the First Ecosystem
- Probable Time for Origin of Life
- Origin of Basic Bio-molecules
- Polymerization and Condensation of Monomers
- Development of Protenoids
- Origin of Organised Structure for Life
- Coacervates (By Oparin)
- Microsphere (By Fox)
- Origin of Selection
1. Introduction to Origin of Life:
Since the development of self consciousness, man has tried to know .the source of origin of life on the earth. Different explanations on the origin of life are inscribed in the books of ancient civilizations, like Greek, Hindu etc. It is interesting to note that till date the phenomenon of origin of life has remained controversial.
At present, several theories have been propounded. These theories will be discussed in brief later on. In this chapter only chemical basis of origin of life will be discussed. In this context it is necessary to know the definition of life and the probable time of its origin, although both are still questionable.
2. Definition of Life:
ADVERTISEMENTS:
Defining ‘Life’ is probably the most problematic task. Numerous scholars attempted to define ‘life’ but neither science nor philosophy has got any success.
However, some of the popular definitions are given below:
i. Ecological Definition:
This definition was proposed by Onsegar-Morowitz and later critically examined and accepted by C. E. Folsome (1979).
ADVERTISEMENTS:
“Life is that property of matter that results in the coupled cycling of biomolecules in aqueous solution, ultimately driven by radiant energy to attain maximum complexity.”
ii. Biological Definition:
According to Strickberger (1996), “the capability of performing various organismic functions such as metabolism, growth and reproduction of genetic material can be designated as life.”
According to Misra (1992), “The life is neither the structure, nor the reproduction, not even metabolism. These are the expression or manifestations of life.”
3. Ancestral Home of life – the First Ecosystem:
ADVERTISEMENTS:
The question of organisation of first life is still debatable. However, whatever may be the initial biological organisation, minimum set of conditions and materials necessary for origin of life is a must. Originating organisms require an elemental and energetic exchange from the non-living world. There are many hypotheses proposed for the possible birth place of life.
These are as follows:
i. Life originated in the sea. This hypothesis is nullified on the argument that in such an open vast place, the original structure would soon be destroyed by strong agents, like various cosmic rays, before it could make new daughter molecules.
ii. According to Darwin, life originated in warm little pond. This was highly criticised by Folsome (1979) and argued that instead of warm pond it will be ‘cold little pond’.
iii. Other ecological combinations like, volcano in a glacial setting or under an ice cap, aerosol droplet in the atmosphere was also proposed as possible ancestral home.
iv. The most important and reasonable hypothesis is that ‘a typical tidal mud flat setting with continuous to and fro flow of rain water and ground water will be the first ecosystem.’ This combination may possibly have provided the energy source to the ancestral life. In 1991, Nisbet proposed that ‘sub-aerial hydrothermal system in a shallow water, in which RNA world could have lived.’ This proposal gained immediate acceptance.
4. Probable Time for Origin of Life:
The Universe has originated through a Big Bang which took place around 15 billion years (Gyr) ago. According to fossil evidence, geologists and paleontologists suggest that cellular life on earth originated within a span of 500 Myr (millions year) between 3.9 and 3.4 Gyr ago (Schopf and Klein, 1992).
5. Origin of Basic Bio-molecules:
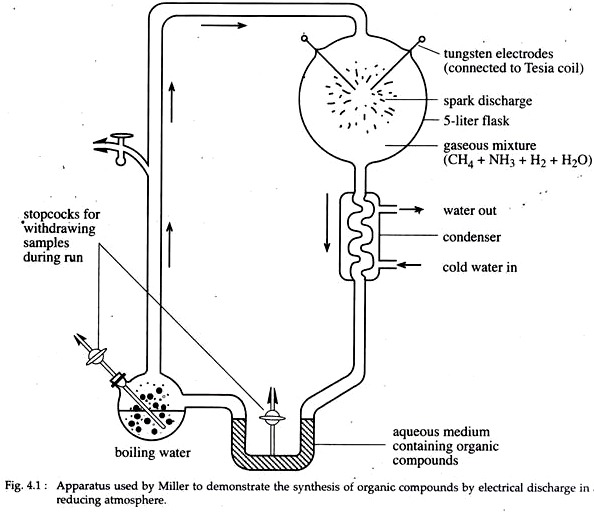
Oparin and Haldane Hypothesis (1924, 29): The primitive atmosphere of the earth was reducing. The organic compounds formed in such an atmosphere might be similar to those presently used by living organisms. An experimental test of this hypothesis was performed by Urey and his student Miller in 1953.
Miller’s Experiment:
ADVERTISEMENTS:
In a glass apparatus (Fig. 4.1), methane, ammonia and hydrogen gases were placed together. An electric spark was generated in the flask. Water was boiled in another flask to provide vapour to the spark as well as to circulate the gases.
Compounds formed by sparking were then condensed or recirculated if they were volatile. After one week of continuous discharge, the accumulated products in the aqueous phase were analysed.
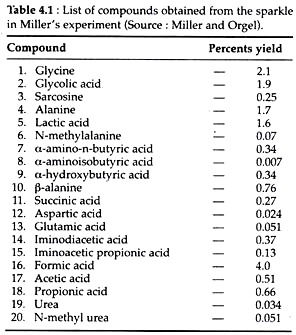
Observations:
Various organic compounds were obtained torn the aqueous phase. They are listed in the table 4.1.
Conclusion:
Large portion of these compounds are relatively simple and include both amino acids and urea, which are found in living organisms. It is remarkable that significant amount of such relatively simple compounds were essential for the formation of life.
This experiment points strongly to the likelihood that the chemical environment that existed before the origin of life was probably not ‘chaos’. Rather, the earth possessed a significant portion of simple organic molecules that could participate in the formation of living organisms, e.g., amino acids, sugars, purine and pyrimidine bases, and fatty acids.
Proposed Steps for Formation of Different Simple Biomolecules:
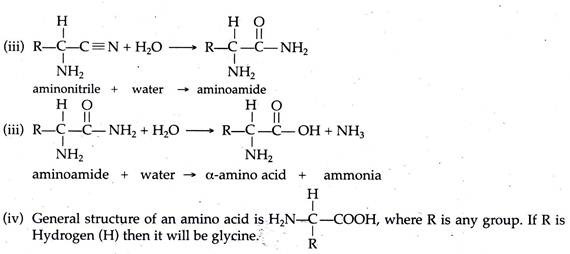
A. Amino Acids:
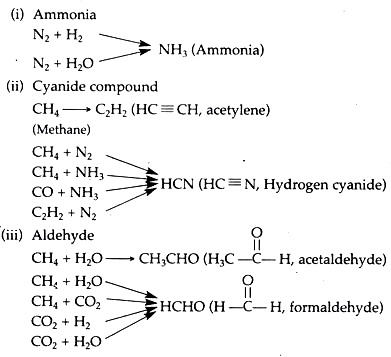
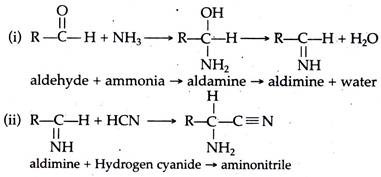
It has been suggested that the amino acids synthesized under primitive earth condition arose primarily from the formation of aldehydes, i.e., R—C—H (where R may represent any group). This aldehyde then interacted with ammonia and cyanide compounds.
All the above mentioned chemicals may have been produced from a variety of simple gases or from their interactions. Probable steps of ammonia, cyanide compounds and aldehyde formation are described below.
From these basic compounds amino acids were synthesized. One of the possible pathways of amino acid synthesis, as proposed by Strecker (known as Strecker synthesis), is as follows.
All the 20 amino acids presently used in protein synthesis of living organism possess a similar structural pattern. Although many of them are synthesized by different bio-chemical pathways.
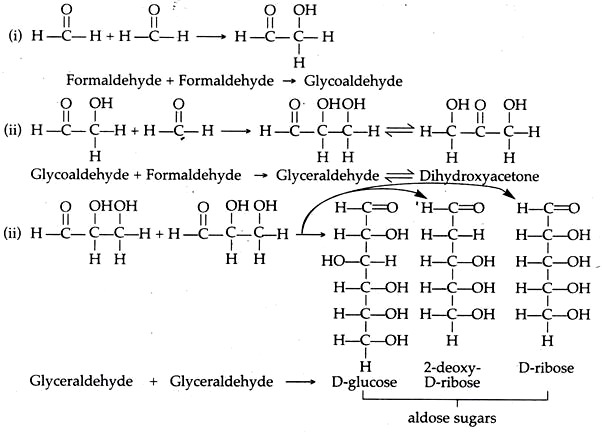
B. Sugars:
These can be synthesized in a fairly simple condition. One of the examples is the condensation of formaldehyde.
The steps are as follows:
Similarly, ketoses sugars are formed from dihydroxyacetone.
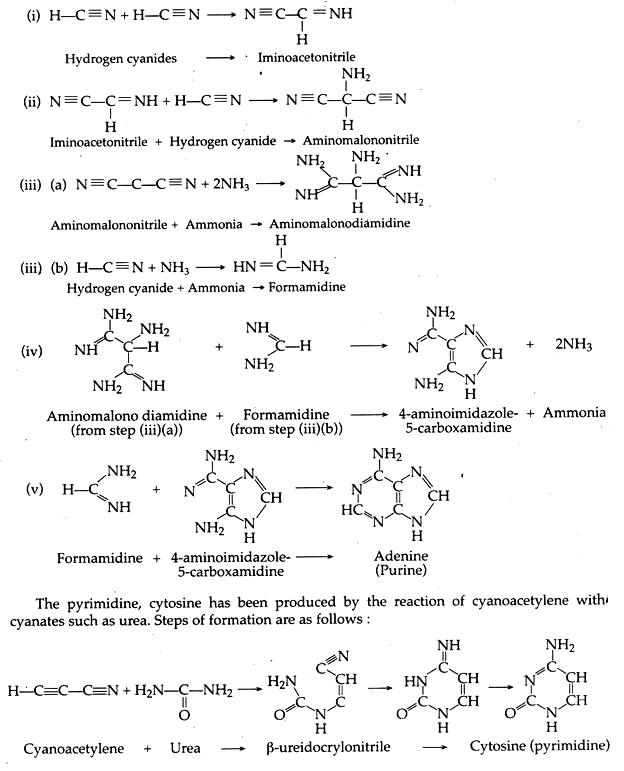
C. Purine and Pyrimidine Bases:
These are essential components of nucleic acids. Oro’ and co-workers have shown that the heating of aqueous solutions of ammonium cyanide can produce up to 0.5 per cent of adenine, a purine. Action of ultra-violet rays on hydrogen cyanide can produce a number of purines, including adenine and guanine.
The steps of condensation of hydrogen cyanide can be described as follows:
Similar procedures have been proposed for the other nitrogenous bases required for the formation of nucleic acids.
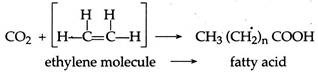
D. Fatty Acids:
Under high atmospheric pressure, carbon dioxides and ethylene molecules can combine to form fatty acids. During the process, y-rays act as an energy source.
In the foregoing discussion, formations of monomers of basic biomolecules are described. But in a living body these molecules remain in different polymeric forms of respective molecules. Therefore, further chemical evolution depends on the polymerization of these monomers, like peptides, polysaccharides, lipids, nucleic acids and so on.
6. Polymerization and Condensation of Monomers:
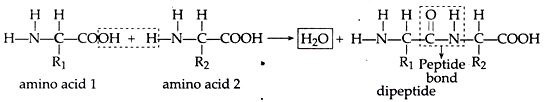
A. Condensation and Polymerization of Amino Acids:
Amino acids are condensed to form polypeptide by peptide bond. When two amino acids condenses it forms dipeptide, e.g.,
Further more and more amino acids condense to form polypeptide chain in the same process.
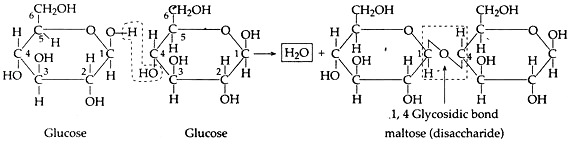
B. Condensation and Polymerization of Sugars:
Two monosaccharide’s condense to form disaccharide by glycosidic bond. Several monosaccharide’s join to form polysaccharides.
Further more and more glucose can condense by this process to form starch (polysaccharide).
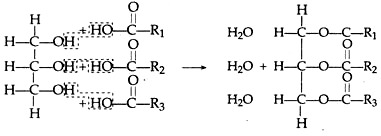
C. Condensation of Fatty Acids and Alcohols:
Fatty acids and glycerol’s (alcohol) combine to form triglycerides. Lipids are the polymers of different triglycerides, e.g.,
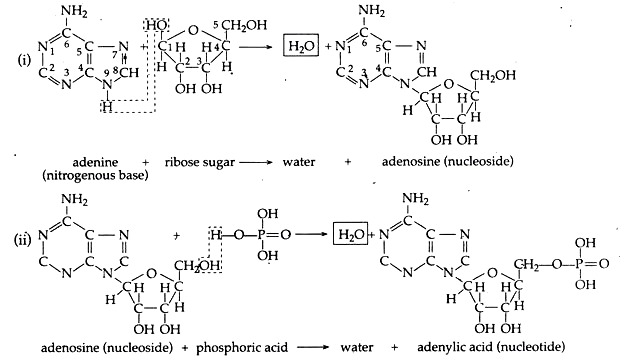
D. Condensation of Nitrogenous Base, Ribose Sugar and Phosphoric Acid to form Nucleotide:
In this process a nitrogenous base combines with a ribose sugar to form nucleoside. Then a nucleoside joins with a phosphate group to form a nucleotide. Different nucleotides polymerize to form nucleic acids (DNA, RNA).
All the polymerization and condensation reactions, as discussed above, depend upon the removal of water molecules. It is also established that all these reactions require energy. Therefore, in prebiotic earth there must be some condensing agents which combine with water and release energy during its hydration process.
So far, compounds identified as condensing agents that could have existed early in the earth’s history include cyan-amide, cyanogen, dicyanamide, dicyandiamide, cyanic acid, cyanoacetylene etc.
7. Development of Protenoids:
During 1950s S. W. Fox and others, developed a technique to produce peptides by heating mixtures of amino acids. These peptides showed some characteristics of proteins in different tests. But, these have gross irregularity in structural configurations i.e., they were, not similar in any aspect to the present day proteins.
Fox and others coined these peptides as protenoids. One of the important characters of these protenoids was its enzyme like activities, which can increase the rate of various organic reactions. Therefore, it was proposed that these enzymes catalyse different reactions for formation of different complex molecular structures, which were necessary for an organism.
It is a matter of debate whether the thermal synthesis of proteins could occur, because exact conditions on the primitive earth are not known. In any case, a wide enough array of condensation mechanisms have been established, one or more of which probably occurred in the past.
Moreover, from the existing information’s, it can be concluded that available cyan-amides, heat, clays and other condensing agents make it highly probable that polypeptides, polysaccharides, lipids and perhaps even polynucleotides were present early in the earth’s history. These polymers then got organised to form a definite structure for the first life on the Earth.
8. Origin of Organised Structure for Life:
Primary life processes are performed by the monomers and polymers of different biomolecules when they remain in a highly organised condition. At its earliest, interactions between molecules must have led them to assume relative positions based on forces such as hydrogen bonding, ionization, solubility, adhesion, surface tension and so on.
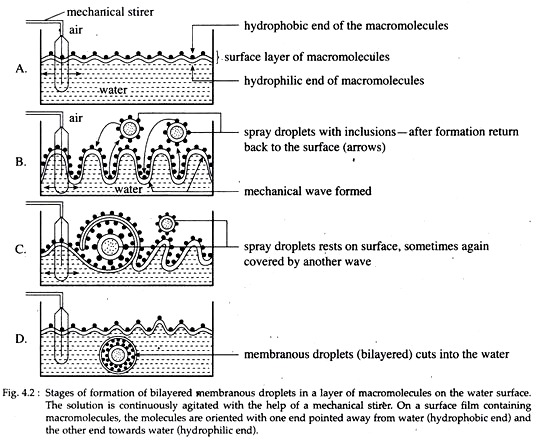
Membranous droplets or vesicles composed of lipids, polypeptides or other molecules were formed in great quantities by the mechanical agitation of molecular films on liquid surfaces (Fig. 4.2). The development of droplet like structure is the most important step towards the origin of life for a number of reasons. The reasons are as follows.
(i) Membrane, surrounding the droplet can selectively control the entry and exit of different compounds within it, i.e., selective permeability.
(ii) Selective permeability will cause certain molecules to accumulate inside the vesicle, which ultimately produce preferred compounds by preferred reactions.
(iii) The small size of the droplet can permit a chain or network of reactions to occur, the products of one reaction can serve as the substrate for others.
(iv) Localized concentration of different molecules within the droplet and their organisation form compartmentations and substructures.
However, the membranous droplets obviously were not able to grow and perform metabolism as well as reproduction as found in present day organisms. But it can be assumed that these droplets will advance one step towards life if they develop the ability to grow i.e., increase in size at least in certain level.
To confirm the basic properties of such vesicles, Oparin and Fox independently produced in their laboratory coacervates and microspheres, respectively.
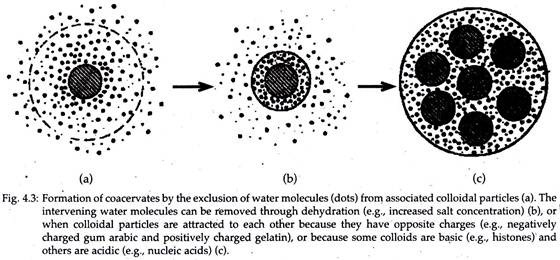
9. Coacervates (By Oparin):
Dispersed colloidal particles separate spontaneously in a solution. They form droplets because of special conditions of acidity, temperature, etc. These droplets are called coacervates (Fig. 4.3). If the solution contains more than one type of macromolecular particles in colloid form, then complex coacervates can be formed.
It shows a number of interesting properties:
1. They possess a simple but persistent organisation.
2. Some coacervates can remain stable in solution for a considerable period.
3. They can increase in size.
Oparin first attempted to search functional ability of these coacervates by incorporating enzymes along with respective substrates into the solution, e.g., synthesis and hydrolysis of starch, synthesis of polynucleotides, etc. He and co-workers observed that at least in some cases the enzyme can function at least to some extent.
Example:
In a coacervate system, chlorophyll irradiated with visible light was placed. If reduced ascorbic acid and oxidized methylene red is dissolved into the solution, these will continuously take entry into the chlorophyll.
Within the droplets these are converted into oxidized ascorbic acid and reduced methylene red, then comes out. Chlorophyll picks up electron from ascorbic acid then supplies to the mythelene red reduction — a process, similar to common non-cyclic photosynthesis.
10 .Microsphere (By Fox):
During the preparation of protenoids, Fox (1984) observed some small spheres in the boiled water. He allowed these spheres to cool and called them microspheres. These microspheres are uniform in size, stable, bounded by double membranes that appear somewhat cell-like and can undergo fission and budding.
In microspheres, active internal physiological processes like selective absorption and diffusion of certain chemicals were observed.
The spontaneous self-assembly of bio-molecules into coacervates or microspheres indicates that such type of organisation may appear in prebiotic atmosphere. But simultaneously, it is clear that these structures have to undergo huge evolutionary changes to become a present day cellular entity.
It is probable that such evolutionary changes must have occurred, and it is also possible that varieties of such organisations had appeared on earth which was ultimately subjected to selection.
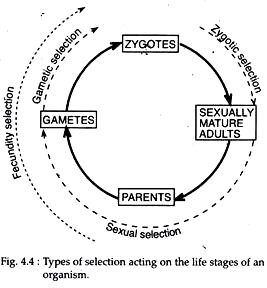
11. Origin of Selection:
Primitive structures, either coacervates or microspheres, were sharing one important evolutionary feature, i.e., they served as the first distinctive, multi-chemical individuals, or proto-cell, that could interact as units with their environment. Together with their various neighbours and progenies, such individuals would form a group or population, upon which natural selection had acted.
Therefore, a proto-cell having confident organisation and metabolic activities to support successful growth and division was selected by nature. Ultimately, this proto-cell population increased mostly in relative frequency, in the area it occupied.
Then gradually multiplied and organised itself to form a successful living organism. Now-a-days it is beyond argument that an organism faces several types of selection during its life stages, which starts from the zygote (Fig. 4.4). In evolution zygote is considered to be the evidence for protozoan ancestry i.e., it may be proto-cell.