In this article we will discuss about the Gastrulation Process in Animals:- 1. Meaning of Gastrulation 2. Activities of Gastrulation 3. Concept of Potency and Totipotency 4. Various Animals 5. Molecular Basis in Early Avian Development.
Contents:
- Meaning of Gastrulation
- Activities of Gastrulation
- Concept of Potency and Totipotency in Gastrulation
- Gastrulation in Various Animals
- Molecular Basis in Early Avian Development
1. Meaning of Gastrulation:
The term gastrulation is applied to the process which produces the gastrula (Greek: gaster, stomach). It is a process of highly coordinated cell and tissue movement whereby the cells of the blastula are rearranged to form the-three germ layers ectoderm, mesoderm and endoderm.
ADVERTISEMENTS:
The numerous cells of the blastula formed due to cleavage, are given new positions and neighbours during gastrulation resulting in the establishment of the multi-layered body plan of the adult organism. During gastrulation the blastocoelic cavity is obliterated and a new cavity called archenteron is formed.
Gastrulation can be defined “as the dynamic process during which the major, presumptive organ-forming areas of the blastula become rearranged and reorganized in a way which permits their ready conversion into the body plan of the particular species” (Nelsen, 1953).”
Thus, gastrulation is the embryo’s way of laying down its body plan. Gastrulation is a well-integrated process, controlled largely by intrinsic (i.e., autonomous) forces. These internal forces in turn are correlated with external conditions.
2. Activities of Gastrulation:
ADVERTISEMENTS:
Thus, the activities of gastrulation are subdivided as follows:
I. Morphogenetic movement of cells.
II. The organization centre and its organizing influences.
III. Chemo-differentiation.
ADVERTISEMENTS:
I. Morphogenetic Movement of Cells:
Morphogenetic movement may be defined as the movement of cells from one place in the embryo to another, to establish a particular form or structural arrangement.
It is of the following types:
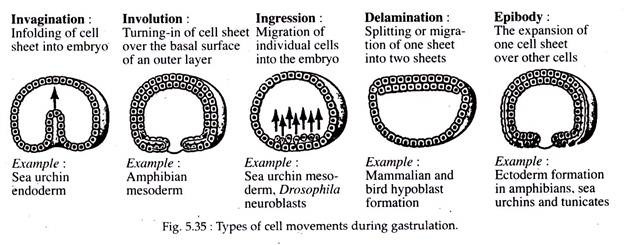
(a) Epiboly:
The word epiboly is derived from a Greek word which means throwing on or extending upon. Epiboly (Fig. 5.35) otherwise known as ectodermal expansion involves the movements of the presumptive epidermal and neural areas during the process of gastrulation.
These two presumptive areas undergo general migration on the surface, in the direction of the anteroposterior axis of the future embryonic body. In simpler words it may be said as the expansion of one cell sheet over other cells.
(b) Emboly:
The word emboly is derived from a Greek word meaning to throw in or thrust in. Emboly refers to the inward movement of the presumptive chorda-mesodermal and endodermal areas (refer fate map page 540) and their extension along the anteroposterior axis of the future embryo. This inward movement of cells is due to innate forces within various cell groups.
ADVERTISEMENTS:
Embolic movement (Fig. 5.35) involves the following types of cell behaviour:
(i) Invagination:
It implies an in-folding or in-sinking of a sheet of cells into the embryo. It results in the formation of a cavity surrounded by these in-folded cells. This process is executed in two ways:
a. Mechanical or passive in-folding of cells as seen in the lateral and ventral lip areas of the blastopore.
b. Active inward streaming or in-pushing of cells as exhibited by the dorsal lip region of the blastopore, into the blastocoelic space.
(ii) Involution:
The word involution means a “turning in” or inward rotation of cells. Involution is dependent much upon the migration of cells toward the blastoporal lip. These cells instead of piling up along the outer edges of the blastoporal lip or along the primitive streak, tend to move over the lip to the inside edge of the lip and are thus deposited on the inside of the embryo along the inner margin of the blastopore.
(iii) Convergence and Divergence:
The migration of cells from the outside surface of the blastula to the external margin of the blastoporal lip is called convergence. Divergence is the opposite of convergence, where the cells diverge after getting aggregated. For example, the cells having involuted over the blastoporal lips into the interior, migrate away from that position to their future positions within the forming gastrula.
(iv) Concrescence:
Concrescence, the term used in older descriptions of gastrulation, denotes the movement of masses of cells toward each other, particularly in the region of the blastopore. It is used to imply the idea of fusion of cell groups. It probably does not occur.
(v) Cell Proliferation:
Cell proliferation implies an increase in the number of cells. It is intimately associated with the gastrulative process in Amphioxus, while in frog it is of lesser importance.
(vi) Ingression:
When a cell or small groups of cells separate itself from other layers and migrate into the segmentation cavity within the developing body; then it is termed as ingression or poly-invagination. This is seen in the case of reptiles, birds and mammals where the mesodermal cells detach themselves from the primitive streak and migrate into the space between the epiblast and hypoblast.
(vii) Delamination:
Delamination is the splitting or separation of one sheet of cells either into two sheets or separation from other cell groups. The separation of notochordal, mesodermal and endodermal tissues from each other, during gastrulation of frog, to form discrete cellular masses, is an example of delamination. Another example, is the formation of hypoblast in mammalians and birds.
(viii) Extension:
The extension of cellular masses also takes place in gastrulation. For example, the extension of the presumptive neural and epidermal areas externally and of the notochordal, mesodermal and endodermal cells after they have moved inward beneath the neural plate and epidermal material.
II. Chemo-Differentiation:
As the late blastula converses into the late gastrula, the presumptive neural plate ectodermal and epidermal ectodermal areas become changed physiologically, as a result they no longer are determined in a presumptive sense but have undergone changes which make them self-determining. This change is called determination and the biochemical change which effects this alteration is known as chemo-differentiation.
As chemo-differentiation involves physiological changes, it restricts changes in potency upon many localized cellular areas. As a result various future organs and parts of organs have their respective fates rigidly and irrevocably determined at the end of gastrulation. Chemo-differentiation apparently occurs through inductive action.
3. Concept of Potency and Totipotency in Gastrulation:
Potency is referred to that property of a cell which enables it to undergo differentiation. Potency is defined as the power or ability of a cell to give origin to a specific kind of cell or structure or to various kinds of cells and structures.
Totipotent, the word coined by Wilhelm Roux, refers to the power or ability of an early blastomere or blastomeres of a particular animal species to give origin to the many different types of cells and structures characteristic of the individual species.
To describe the ability of a cell, it is well to define two kinds of totipotency — totipotency and harmonious totipotency. The former term refers to the ability of a cell or cell group to give origin to all or nearly all the different cells or tissues of the particular species to which it belongs, but it lacks the ability to organize these cells or tissues into a harmonious organism.
Harmonious totipotency possesses the above of potency and in addition it has the power to develop a perfectly organized body. A fertilized ovum has the capability to form an entire organism and this capability is retained by the individual cell resulting from the first few divisions after fertilization, in many vertebrates. Such cells are said to be totipotent.
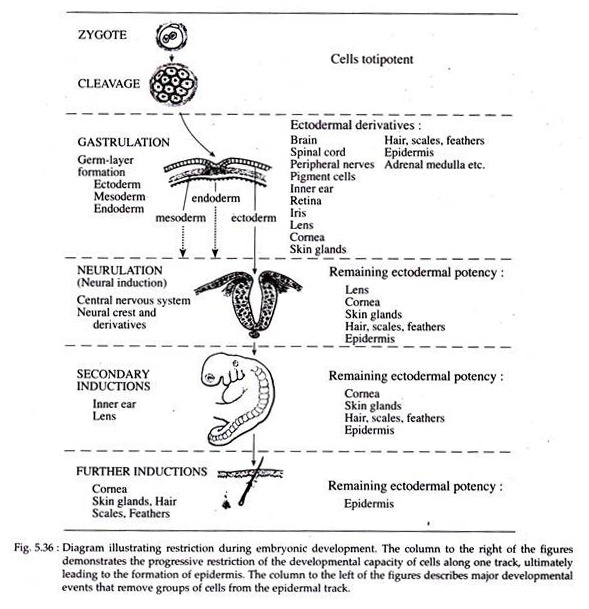
As development proceeds, the cells gradually loose the ability to form all the types of cells that are found in the adult body. This reduction in the developmental options permitted to a cell is called restriction. A general pattern of restriction during development is given in Fig. 5.36.
Cleavage leads to the formation of the blastula which then undergoes gastrulation to form the three primary germ layers. This results in the formation of an outermost ectodermal layer, a middle mesodermal layer and an innermost endodermal layer.
By this time at least one stage of restriction has taken place and the cells of the three germ layers are now locked into different developmental channels (Fig. 5.36) and thus have lost their totipotency and are no longer interchangeable.
Further, as development proceeds, the cells of the ectoderm becomes thickened and becomes committed to forming the brain, the spinal’ cord and other associated structures. The remainder of the ectodermal cells can no longer form these structures and have thus undergone another phase of restriction.
When restriction ultimately results in a group of cells becoming committed to a single developmental fate (e.g., formation of cornea), then the determination of these cells has taken place.
The word determination is applied to those unknown and invisible changes occurring within a cell or cells which effect a limitation or restriction of potency. Often, tissue interactions called inductions take place shortly before determination.
4. Gastrulation in Various Animals:
I. Gastrulation in Amphioxus:
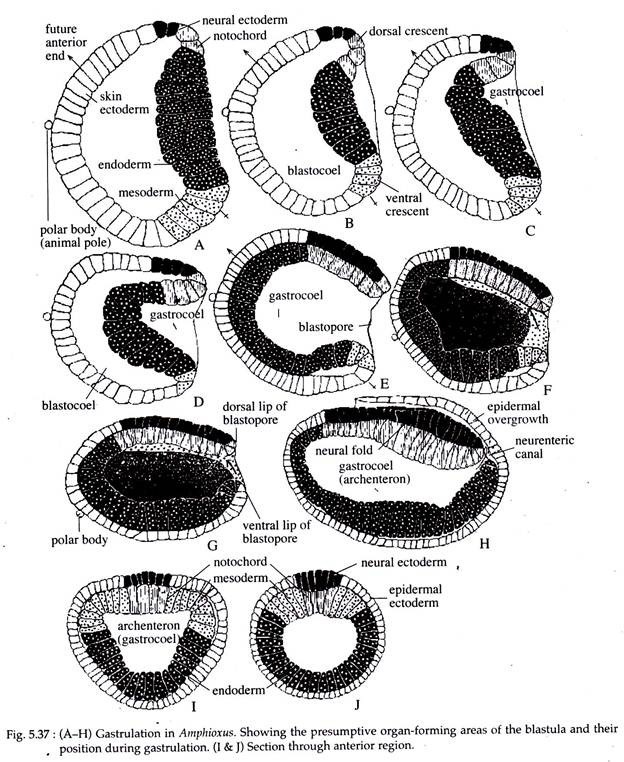
Gastrulation in Amphioxus starts when the embryo reaches the 800 cell stage. Gastrulation in Amphioxus involves both epiboly and emboly. In emboly the processes involved are invagination, cell proliferation, involution, extension, convergence and constriction of the blastopore.
Prior to the studies of gastrulation, consult the fate map and blastulation of Amphioxus for the position of the micromeres and macromeres and the presumptive organ forming areas.
Gastrulation in Amphioxus consists of the following morphogenetic movements:
(a) Emboly:
At the onset of gastrulation an increased mitotic activity occurs in the crescentic area, comprising of the presumptive notochordal and neural plate cells at the dorsal crescent and the future mesodermal cells at the ventral crescent (Fig. 5.37B).
As these cells are proliferating, the endodermal cells at the vegetal pole, comprising of the mega-meres, gradually start to invaginate or fold inwardly into the blastocoelic cavity (Fig. 5.37). As invagination continues, the upper portion of the endodermal cells move inward more rapidly and pushes forward towards the future anterior end of the embryo.
As this goes on the blastocoelic cavity gets obliterated and a new cavity called archenteron is formed. The archenteron opens to the outside by a small opening called the blastopore.
As the endodermal cells at the initial stage are invaginating, the notochordal cells start involution or rotating inward along the mid-dorsal region of the blastopore. They finally come to occupy the mid-dorsal area of the embryo. At the same time, the mesodermal cells also converge dorsolaterally.
They pass inwardly towards the roof of the anchenteron and occupy the position on either lateral side of the notochordal cells (Fig. 5.37F and G). Thus the roof of the archenteron is formed of notochordal and mesodermal cells (Fig. 5.37 I and J).
(b) Epiboly:
As the different events of emboly are taking place, epiboly also takes place more or less simultaneously. The potential epidermal and neural cells actively proli ferate and gradually extend from the animal to the vegetal pole or in an anteroposterior direction. The neural ectodermal cells, in this way become elongated into a narrow band along the mid-dorsal region of the gastrula (Fig. 5.37). The epidermal area, on the other hand, covers the entire gastrula externally except the areas covered by the neural cells.
Thus, the process of gastrulation results in the formation of a rudimentary double- layered embryo or gastrula, with a central cavity called archenteron that opens to the outside by the blastopore. The entire outer surface of the gastrula is covered by the ectoderm. The internal layer is composed of notochordal cells in the dorsomedian area with two narrow strips of mesodermal cells lying on either side of it and the remainder of the internal layer is occupied by the endodermal cells.
(c) Antero-posterior Extension of the Gastrula:
The process of epiboly and emboly, along with rapid cell proliferation at the blastoporal-lip area, effects an anteroposterior elongation of the developing gastrula (Fig. 5.37H).
(d) Closure of the Blastopore:
At a later stage of gastrulation, the blastopore gradually moves to a dorsal position. As neutralization takes place of the neural ectodermal cells, the epidermal ectoderm of the ventral lip of the blastopore grows dorsally, and that of the lateral lips grow in the median direction. This results in the closure of the blastopore by the coming together and fusion of these ectodermal growths.
Role of blastopore in Embryonic Induction:
That primary organizer and neural induction do exist in the lower chordates and particularly in Amphioxus, has been demonstrated by the experiment of Tung et al. (1962). They transplanted pieces of tissue from the inner surface of the dorsal blastopore lip of an early gastrula of Amphioxus into the blastocoel of another embryo in the same stage.
They observed that a secondary embryo developed in the ventral region of the host with a notochord and mesoderm. This secondary embryo was produced by the graft and a neural tube was induced from the host tissue. Thus, the dorsal blastoporal lip of Amphioxus has embryonic induction properties.
II. Gastrulation in Frog:
Gastrulation in frog is considered to be both one of the oldest as well as one of the newest areas of experimental embryology, as the mechanism of their development has been revised over the past decade. The gastrulation studies has been complicated as a number of morphogenetic movements are involved in it.
Frog undergoes both epiboly and emboly and the latter includes various types of cell movements such as invagination, convergence, involution, divergence, extension and constriction. Prior to the studies of morphogenetic movements involved in gastrulation, one should consult the fate map of frog’s blastula.
The late blastula of frog is composed of the presumptive organ-forming areas arranged around the blastocoelic space. The yolk laden, future endodermal cells form the hypoblast at the vegetal pole, while the rest part forms the epiblast.
Physiological Changes in the Presumptive Organ-forming Areas at the time of Gastrulation:
During the process of gastrulation, the late blastula undergoes a striking physiological change in the presumptive organ-forming areas of the epiblast.
Interchanges of cells from the epidermal area to the neural area or to the mesodermal area and vice versa are possible during the early phase of gastrulation without disturbing the normal sequence of events.
However, such interchanges are not possible at the end of gastrulation. Also, no such physiological changes take place in the hypoblast, i.e., in the presumptive endodermal cells.
Morphogenetic movements:
(a) Position of Blastopore:
The point of sperm entry results in rotating the cortical cytoplasm of the ovum to 30 degrees animally (upward). This produces a new symmetry in the fertilized egg, as it becomes bilaterally symmetrical due to its formation of dorsal-ventral axis.
These cytoplasmic movements activate the cytoplasm opposite to the point of sperm entry in initiating gastrulation. It will form the blastopore and becomes the dorsal portion of the body. If the cortical rotation is blocked, there is no dorsal development and the embryo dies as a mass of ventral (primarily gut) cells.
The sperm does not induce these movements in the egg cytoplasm, but is important in determining the direction of the rotation.
(b) Emboly:
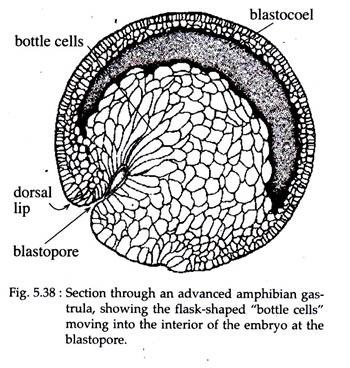
(i) Invagination and Involution:
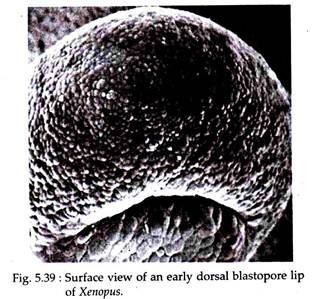
Gastrulation in Xenopus is initiated when a group of marginal endodermal cells, just below the gray crescent area on the dorsal surface, undergoes invagination by folding inward and forward, towards the future cephalic end of the embryo (Fig. 5.38). The outer or apical surfaces of these cells contract dramatically, while their inner or basal ends expand.
The apical-basal length of these cells increases tremendously and acquires the structure of ‘bottle’ shape. The main body of each bottle shaped cell is displaced towards the inside of the embryo while the cell maintains contact with the outside surface by way of a slender neck (Fig. 5.39).
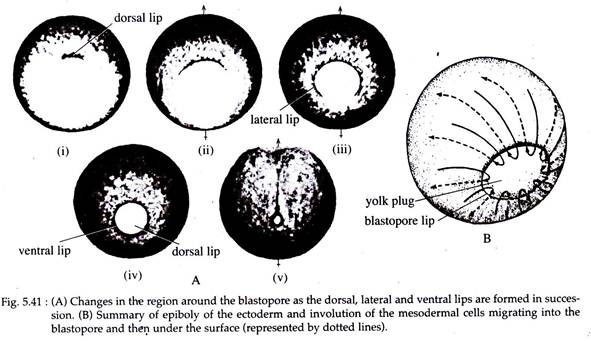
The surface pull thus exerted, results in the formation of a slit- like dorsal lip of the blastopore. The bottle cells, which are so prominent in early gastrulation, decrease in height as gastrulation proceeds and ultimately become indistinguishable from other endodermal cells of the endodermal lining.
Hardin and Keller (1988) have shown that the bottle cells of Xenopus although plays a role in initiating the involution of the marginal zone, they are not essential for gastrulation to continue. This has been experimentally proved that when these bottle cells are removed after their formation, involution, blastopore formation and its closure still continues.
After the initial dorsal lip is formed, involution begins dorsally. Much of the process of gastrulation consists of surface cells moving into the interior of the embryo through the blastopore. As cells turn inward or involute around the lips of the blastopore, they are followed by other cells which move over the surface of the embryo towards the blastopore.
As the endodermal cells migrate inward, the prechordal plate cells followed by the notochordal cells involute to the inside.
(ii) Convergence, Extension and Divergence:
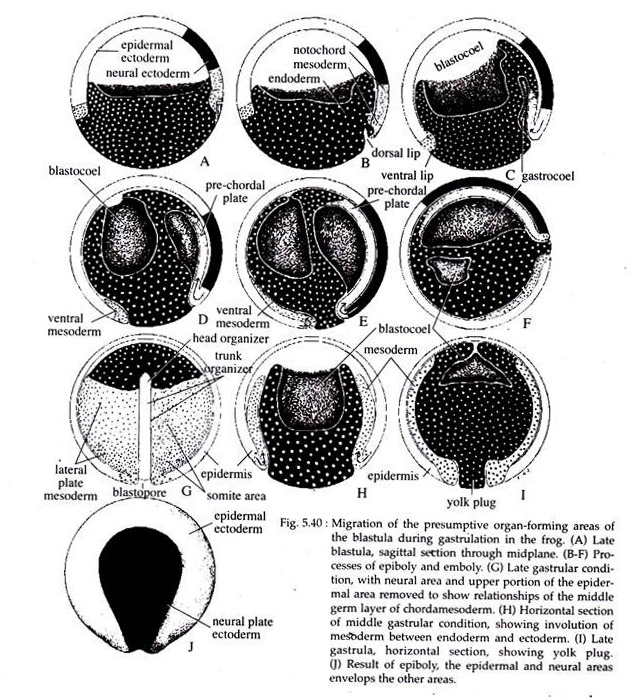
The more laterally situated notochordal cells converge towards the dorsal lip and gradually pass to the inside and lie in the mid-dorsal region of the embryo. Here, the notochordal cells undergo extension anteroposteriorly and form a narrow band of cells below the neural plate area and on the dorsal side of the embryo (Fig. 5.40 C-F).
As gastrulation proceeds, the endodermal cells move more inwards. As the endodermal cells below the site of invagination begins to rotate inwards, the dorsal blastoporal lip starts widening. At the same time the presumptive somatic mesoderm, located externally along either side of the notochordal area, migrates or converges towards the forming dorsolateral lips of the blastopore.
They then undergo involution over the lip, and position themselves between the inside endoderm and the external surface layer of cells (Fig. 5.40H). The dorsal lip of the blastopore thus undergoes lateral extension and forms the lateral lips.
As the lateral lips continue to form and extend towards the ventral direction, they eventually form the ventral lip of the blastopore. With the formation of the ventral lip, the blastopore has formed a ring around the large endodermal cells that remain exposed on the surface of the vegetal area. This remaining patch of endoderm is called the yolk plug (Fig. 5.40 and 5.41).
The formation of the ventro-lateral and ventral blastoporal lip is associated with convergence and involution of the ventro-lateral and ventral mesoderm of the gastrula (Fig. 5.40 D and F). The yolk plug eventually internalizes and accompanying this, is an inward rotation of the endodermal mass. This results in a counter clockwise rotation of the endodermal organ-forming rudiments.
In this way the organ-forming areas of the endoderm get arranged, with the foregut material being now situated toward the anterior end of the developing embryo, while the stomach, liver, small intestine and hind gut regions are placed progressively posterior ward with the hind gut area near the closing blastopore. The tail mesoderm converges toward the dorsal blastoporal area when the blastopore nears closure.
The early archenteron at the time when involution has just started, is lined by chorda-mesoderm on its dorsal surface and the rest by endoderm. As involution continues, the archenteron increases, in size and extends beneath the outer layers of cells toward the cephalic end of the embryo. In so doing they gradually obliterate the blastocoelic cavity.
(c) Epiboly:
While involution is taking place at the blastopore lips, the ectodermal precursors are simultaneously expanding over the entire embryo. The major mechanism of epiboly in Xenopus gastrulation is an increase in cell number (cell proliferation) along with an integration of several deep layers into one.
The most superficial layer expands by cell division and flattening. It gradually comes to cover the entire external surface of the gastrula with the exception of the immediate blastoporal area (Fig. 5.40A-E). The neural ectodermal area also elongates, but only along the anteroposterior embryonic axis, where it forms a shield shaped region with the broad end of the shield located anteriorly (Fig. 5.40J).
As the ectoderm epibolizes over the entire outer surface of the embryo, it ultimately pushes all the endoderm into its interior. The inward movement of the endoderm and yolk induces a rotation of the entire gastrula (Fig. 5.40E & F).
The blastopore gradually grows smaller and the tail mesoderm becomes located inside the dorsolateral portion of the closed blastopore. At this point, the epidermal and neural ectoderm cover the entire surface of the embryo, the endoderm is located within the embryo and the mesoderm is positioned between them.
Role of Blastopore in Embryonic Induction:
The dorsal lip of the blastopore of frog possesses the ability to ‘organize’ much of the future development of the embryo and this has been ably demonstrated by Spemann and Mangold in 1924. As the dorsal blastoporal lip has the ability to direct normal development in an orderly fashion, Spemann called it the organizer. In frog, the organizer has several specific functions.
Early, it induces convergent extension movements, that characterizes gastrulation. During late gastrulation the organizer exerts a dorsalizing effect on the lateroventral region of the marginal zone, inducing these cells to form somites and kidney tissue. The organizer, finally, signals neighbouring cells of the animal cap to form neural plate (neural induction).
III. Gastrulation in Chick:
The process of gastrulation in chick is peculiar as the egg is telolecithal and no true archenteron being formed. Although chick’s gastrulation is very complicated and a prolonged one, still it gets completed by the second day of incubation.
There exists a lot of discrepencies regarding the range of the process of gastrulation in chick. Ede (1978) regarded the beginning of gastrulation after the formation of area opaca and area pellucida. Balinsky (1981), Carlson (1996), were of the opinion that the formation of hypoblast to be a pregastrular morphogenetic movement.
Here, we consider the formation of epiblast and hypoblast to be the starting point of gastrulation, in accordance with that of Gilbert (2000).
(a) Formation of Hypoblast and Epiblast:
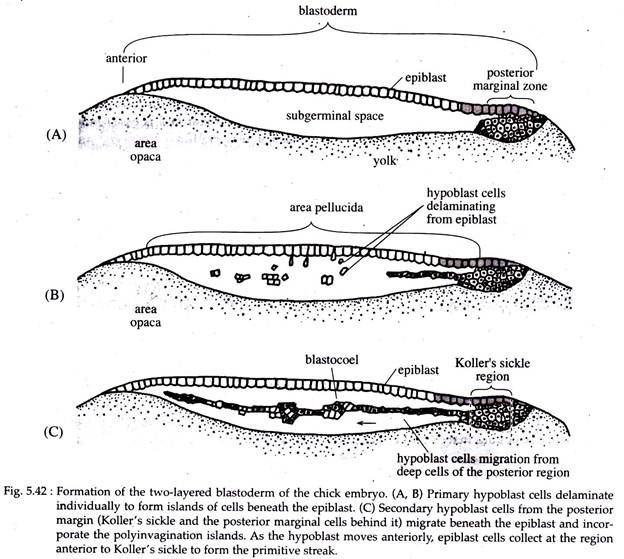
At the time of completion of cleavage and the formation of area opaca and area pellucida, the blastoderm of chick contains about 20,000 cells. Most of the cells of area pellucida remain at the surface and forms the epiblast.
A few remaining cells get delaminated and migrate individually into the sub-germinal cavity to form the poly-invagination islands (primary hypoblast). It comprises of separate clusters, each consisting of 5-20 cells (Fig. 5.42B).
Shortly thereafter, a thin sickle-shaped mass of cells (called Roller’s sickle) take shape at the posterior end of the embryo. From these Koller’s sickle, (Fig. 5.42C) a second generation of hypoblast cells push anteriorly forming the secondary hypoblast.
These cells compress and fold the primary hypoblast ahead of it. The two layered blastoderm comprising of the epiblast and hypoblast joined together at the margin of the area opaca and the space between the layers forms a blastocoel.
The embryo of the chick is formed entirely from the epiblast. The hypoblast does not contribute any cells to the formation of the developing embryo. It rather, forms portion of the external membranes, especially the yolk sac and the yolk stalk that links the yolk mass to the endodermal digestive tube.
(b) Fate Map:
All the three germ layers of the embryo proper is formed by the epiblastic cells.
(c) Formation of Primitive Streak:
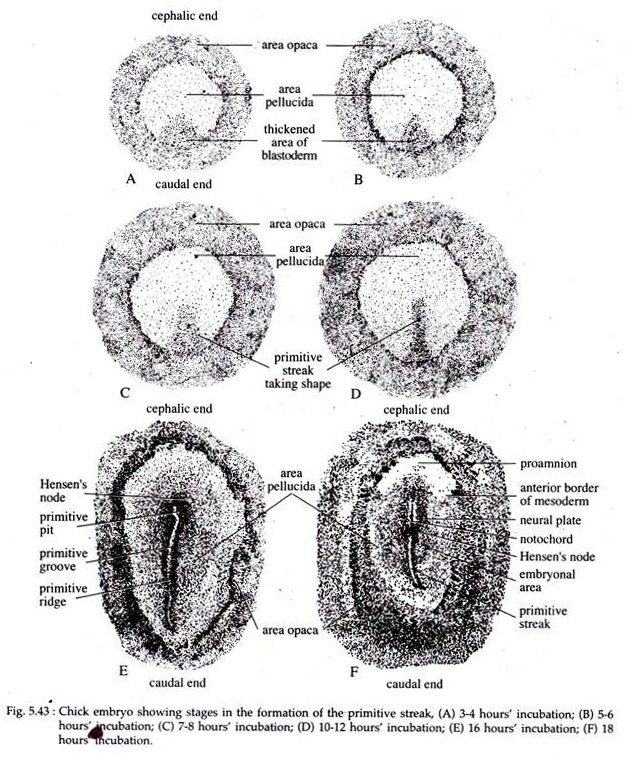
The formation of primitive streak is a major structural characteristic, not only in avians but also in reptilian and mammalian gastrulation. At first a visible thickening of the epiblast at the posterior region of the embryo, just anterior to Koller’s sickle, appears at about 3 to 4 hours of incubation (Fig. 5.43A).
This thickening is caused by the condensation of cells which gradually elongate cephalocaudally and by 10 to 12 hours of incubation the thickened area (Fig. 5.43) assumes a shape called the primitive streak.
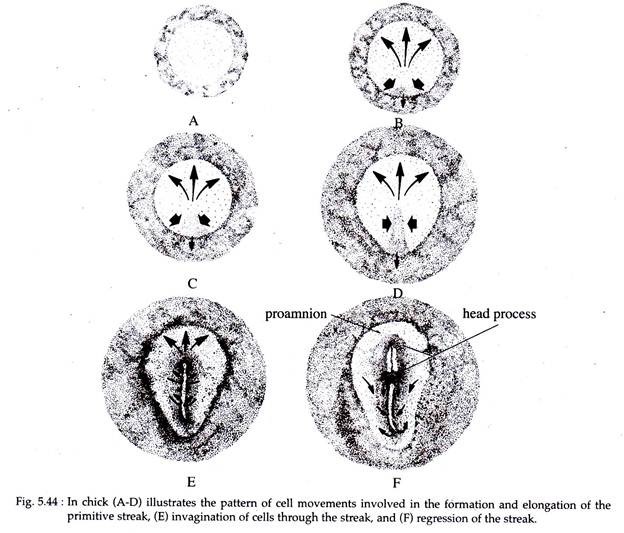
According to Eyal-Giladi and Wolk (1970), the appearance of the primitive streak is due to an inductive interaction of the epiblast with the hypoblastic layer. The early primitive steak elongates initially in both cephalic and caudal directions.
Cells from the lateral areas then converge towards the primitive steak (Fig 5.44). As more and more cells enter the streak region, it elongates in the cephalic direction. Caudal extension of the primitive streak also takes place and it moves into the area opaca.
After about 16 hours of incubation the primitive streak becomes very prominent (Fig. 5.43E). It is characterized by a central furrow called the primitive groove. The primitive groove is flanked on both the sides by thickened margins called the primitive ridges.
The cephalic end of the primitive streak has closely packed cells which form a local thickening known as the primitive knot or Hensen’s node. The centre of this node contains a funnel-shaped depression called the primitive pit (Fig. 5.43E).
The thickened area around the primitive streak constitute the embryonal area at about 18 hours incubation (Fig. 5.43F). Due to its shape this embryonal area is often referred to as the embryonic shield.
(d) Migration of Endoderm and Mesoderm Cells through the Primitive Streak:
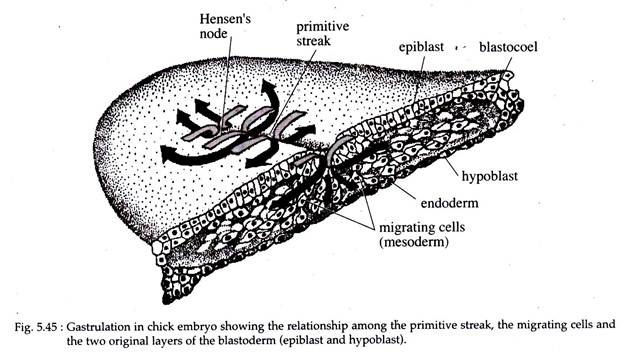
In chick, gastrulation takes place by the coordinated passage of individual cells from the exterior to the interior of the embryo by ingression. As soon as the primitive streak is formed the epiblast cells start to migrate through it and into the blastocoel.
The primitive streak has a population of cells that are continuously changing, as the cells converging on the primitive streak moves inward, their place is taken up by another batch of cells.
The first group of cells to migrate through the Hansen’s node are the presumptive endodermal cells that are destined to form the foregut. These cells pass down into the blastocoel, migrate anteriorly and eventually displace the hypoblast cells (Fig. 5.45).
The hypoblast cells get confined at the anterior portion of the area pellucida, at a region (edge of area opaca) called the germinal crescent. This germinal crescent does not form any embryonic parts, but it contains the precursors of the germ cells, that later migrate via the blood vessels to the gonads.
The next cells to migrate into the blastocoel through Hensen’s node are the embryonic mesodermal cells, like head mesenchyme and. the precordal plate mesoderm, that also move anteriorly and position itself between the endoderm and the epiblast.
These early-ingressing, anteriorly moving cells push up the anterior midline region of 1 the epiblast to form the head process (Fig. 5.44F). Thus, in chick embryo the head process forms anterior to Hensen’s node.
The presumptive notochordal cells are the next to migrate through the Hensen’s node and they extend up to the presumptive mid brain area where they meet the precordal plate mesoderm. These notochordal cells form the hind brain and trunk at the level of Hensen’s node.
As the above processes are going on, cells also migrate continually inward through the lateral portion of the primitive streak. These cells on entering the blastocoelic cavity separates’ into two layers. The deeper lying cell layer joins the hypoblast cells along its midline and displaces them to the sides.
These deep-moving cells give rise to all the endodermal organs of the embryo and also contribute considerably to the formation of extra-embryonic membranes (the rest being formed by the hypoblast cells).
The second migrating layer of cells move between the above endoderm and the epiblast. These middle lying cells form a loose layer, comprising of the mesodermal portion of the embryo and the extra-embryonic membranes.
By 22 hours of incubation most of the presumptive endodermal cells have moved into the blastocoelic cavity, while the future mesodermal cells still continue to migrate inward. As the above migration of cells are taking place, the area pellucida undergoes a change in shape from an essentially circular disk to a pear-shaped structure (Fig. 5.44D, E and F).
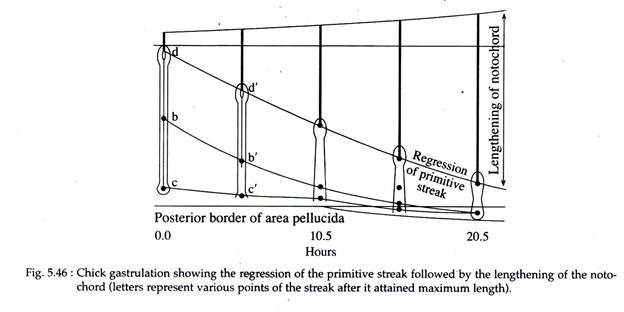
(e) Shortening of the Primitive Streak:
While the presumptive mesodermal cells are still undergoing ingression and after the primitive streak has reached its full length at about the 18th hour of incubation, the streak starts shortening by moving the Hensen’s node from near the centre of the area pellucida to a more posterior position (Fig. 5.46).
Accompanying this shortening of the primitive streak is a corresponding elongation of the notochord. As the Hensen’s node moves posteriorly, the anterior portion of the notochord is laid down starting from the level of the future mid-brain. Then the posterior portion of the notochord is laid down, that extends posteriorly to form the tail of the embryo.
The Hensen’s node shortens further and finally the anterior end of the streak (primitive knot or Hensen’s node) together with some adjacent, condensed streak tissue forms the end bud. At its most posterior position the Hensen’s node forms the anal region. By this time all the presumptive endodermal and mesodermal cells have entered the embryo and the epiblast is composed entirely of presumptive ectodermal cells.
(f) Epiboly:
At the time when the future mesodermal and endodermal cells are ingressing inwards, the presumptive ectodermal cells start proliferating. These cells undergo epiboly and they migrate to enclose the yolk.
The surrounding of the yolk by the ectodermal cells is an enormous task and it takes about 4 days to complete. Epiboly brings about an elongation of the presumptive neural ectodermal area, converting it into an elongated band of cells lying above the notochordal area.
Finally, as the chick’s gastrulation comes to a close, the ectoderm has covered the entire outer surface (including the yolk), the endoderm has replaced the hypoblast and the mesoderm has positioned itself between the ectoderm and endoderm.
5. Molecular Basis in Early Avian Development:
In the molecular aspect of avian development the expression of some embryonic genes begins as early as the morula stage.
Spratt (1946), with his experiment of carbon particles marking in the pre-streak and early -streak stages, had shown that the forward movement of the hypoblast precedes the anterior differentiation of the primitive streak, suggesting that the hypoblast influences the development of the primitive streak on the overlying epiblast.
The molecular side of this inducing and axial determining effect of the hypoblast on epiblast is the finding that activin is expressed in the hypoblast and can induce axial structures (notochord, somites and neural tube) in epiblast.
The goosecoid gene is expressed in Hensen’s node. It is also expressed to some extent in the cells of Koller’s sickle, which ultimately forms the Hensen’s node. Morphogen, a molecule important in morphogenesis, has also been traced in Hensen’s node.