In this article we will discuss about:- 1. Definition of Macrophages 2. Types of Macrophages 3. Role in Immune Response.
Definition of Macrophages:
Macrophages are mononuclear phagocytic leucocytes that play important roles in adaptive as well as in innate immunities. They are the first line of defence against bacterial infections and are indispensable participants in the immunological defence by processing and presenting antigens to lymphocytes.
They arise from monocytes that develop in the bone marrow, circulate in the blood for a day or two, and then migrate through the endothelium of post-capillary venules to take up residence in the connective tissue. There they differentiate into macrophages that have a life span of about two months.
Replacement of tissue macrophages goes on continuously at a slow basal rate, but the monocytes circulating in the blood constitute a very large reserve that can be rapidly mobilised at sites of injury of infection and there transform into macrophages.
Types of Macrophages:
ADVERTISEMENTS:
Traditionally, motile macrophages of varying shapes that wander through the ground substances were referred to as free macrophages. Sessile cells that are stretched out along collagen fibres and have a shape not unlike that of fibroblasts were referred to as fixed macrophages. These terms are now given more appropriate descriptive terms.
Resident macrophages are present at a given site, in the absence of an exogenous stimulus. These are fusiform or stellate cells widely distributed among the bundles of collagen fibres of connective tissue, but they tend to be more abundant in the vicinity of small blood vessels. They possess small, darkly stained nucleus and heterogeneous cytoplasm with vacuoles and small dense granules.
Elicited and activated macrophages:
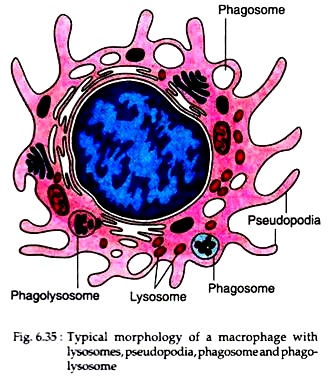
Macrophages that can mobilise to the site of infection in response to stimulus are called elicited macrophages. Macrophages that have acquired enhanced phagocytic and antigen processing activities in response to stimulus are called activated macrophages (Fig. 6.35).
These cells are highly variable in shape. In addition to the pseudopodia that they extend and retract during locomotion, they have many short microvilli and thin undulating folds of their surface called lamellipodia. Such macrophages are much more active in pinocytosis and phagocytosis than the resident macrophages.
These cells may interiorize the equivalent of their total surface area every 30 minutes. They are selective in the kind of particles they ingest.
Epitheloid cells:
In certain pathological conditions, macrophages may have unusual shapes. At sites of chronic inflammation, closely aggregated macrophages may take a polygonal shape owing to their mutual deformation. In this configuration, they are described as epitheloid cells.
ADVERTISEMENTS:
Foreign body giant cells:
When macrophages gather around splinters or other foreign bodies that are too large to be engulfed, they may coalesce to form huge multinucleated masses referred to as foreign body giant cells.
Role of Macrophages in Immune Response:
Macrophages are versatile participants in the body’s immunological defences. The role of macrophages is not limited to disposal of the invaders by phagocytosis, but also interacts variously with lymphocytes in the inductive phase of the immune response.
Phagocytosis:
Macrophages can eliminate a number of substances by phagocytosis and intracellular digestion. Their prey includes whole microorganisms, insoluble particles, injured or dead host cells, cellular debris, activated clotting factors etc. The process of phagocytosis has been described earlier.
Example:
The plasma lemma of macrophages can express some receptors like Fc receptors that bind antibodies and receptors for C3 component of complement. Antibody and complement first bind to the surface of bacteria, making them more vulnerable to phagocytosis by macrophages.
ADVERTISEMENTS:
This process is called opsonisation. When an opsonized bacterium has bound to the surface of a macrophage, ingestion begins with a zipper-like progressive binding of Fc and C3 receptors on its plasma lemma to the corresponding ligands on the surface of the bacterium until the latter is completely enveloped by folds of the macrophage surface.
After membrane fusion at the leading edge of the encircling process, the bacterium, now enclosed in a vacuole (phagosome), is drawn deeper into the cytoplasm. Lysosomes then fuse with the phagosome discharging into it microbicidal peptides and enzymes, including lysozyme that digests the bacterial cell wall and other antimicrobial agents.
The action of these agents and other lysosomal enzymes result in the complete destruction of the bacterium. It is interesting that certain strains of bacteria are resistant to the microbicidal activities of macrophages and therefore, are virulent.
Secretion of antimicrobial and cytotoxic substances:
Activated macrophages produce a number of cytotoxic as well as antimicrobial agents that help in killing of phagocytosed microorganisms. During phagocytosis within activated macrophages, membrane bound oxidase may catalyse the reduction of oxygen to superoxide (O−2) ions that are extremely toxic to ingested microorganisms.
These superoxide ions can again generate other powerful oxidizing agents like hydrogen peroxide (H2O2) and hydroxyl radicals. Fusion of phagosome with lysosomes stimulates myeloperoxidase to generate hypochlorite from H2O2 and Cl− ions. This hypochlorite is also very toxic to ingested microbes.
Macrophages do not act alone in combating infections. They interact with lymphocytes that also gather at sites of bacterial invasion. Lipopolysaccharide (LPS), a cell wall component of Gram negative bacteria and gamma interferon (IFN-y), a cytokine produced by antigen-stimulated T cells activate macrophages to express high levels of nitric oxide synthetase (NOS), an enzyme that oxidizes L-arginine to produce nitric oxide (NO). Nitric oxide has a potent anti-pathogenic activity against bacterial, fungal, helminthic and protozoan pathogens.
Activated macrophages can synthesise a group of peptides, commonly referred to as defensins that can form ion-permeable channels in cell membranes of many bacteria like Staphylococcus aureus, Streptococcus pneumoniae, E. coli etc.
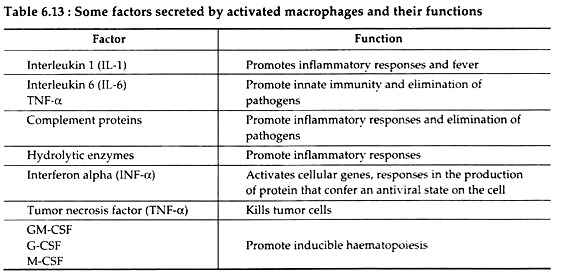
They also synthesise and release interleukins (IL-1, IL-6), tumor necrosis factors (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF), that have wide range of effects on various immune responses (Table 6.13).
Antigen processing and presentation:
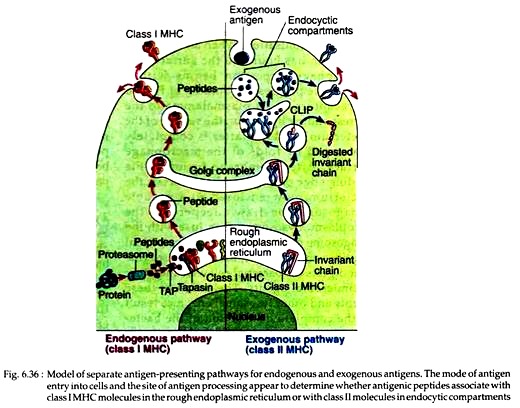
Within activated macrophages, the phagocytosed antigens are digested into peptides, which in association with class II MHC molecules then move to the macrophage membrane and are expressed. However, this occurs through a complicated process. Recent experiments indicate that most class II MHC invariant chain complexes are transported from RER, where they are formed, through Golgi complex to early endosome.
Then from early endosome the complexes move to late endosome and finally to lysosomes. Through this journey, the invariant chain is gradually degraded leaving a short fragment termed as CLIP that remains bound to the class II molecules. This CLIP prevents any premature binding of antigenic peptides with the class II MHC molecules (Fig. 6.36).
During subsequent stages, a non-classical class II MHC molecule, called HLA-DM catalyses the exchange of CLIP with antigenic peptides. This reaction of HLA- DM may be inhibited by HLA-DO. The resulting class II MHC-peptide complexes then move to the plasma membrane of macrophages.