Insects are collected by nets and are killed with potassium cyanide gas slowly released in a capped wide mouth jar. Insects can be mounted and preserved in various ways. Most specimens are pinned, and, once dried, will keep indefinitely. Specimens too small to pin can be mounted on “points,” on tiny “minuten” pins or on microscope slides. Large and showy insects, such as butterflies, moths, grasshoppers, dragonflies or damselflies, may be mounted in various types of glass-topped display cases.
Pinning:
Pinning is the best way to preserve hard-bodied insects; pinned specimens keep well, retain their normal appearance and are easily handled and studied. Insects should be pinned with a special type of steel pin known as an insect pin. Insect pin sizes range from 00 to 7, size 2 and 3 being the best for common use.
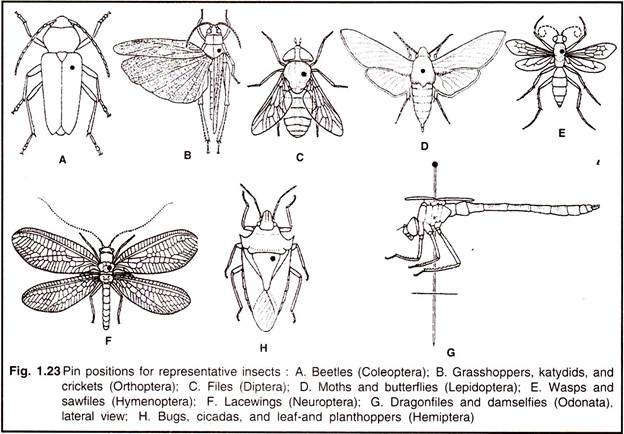
Insects are usually pinned vertically through the body (Fig. 1.23). Bees, wasps, flies, butterflies and moths are pinned through the thorax between the bases of the front wings; with the flies and wasps it is desirable to insert the pin a little to the right of the midline. Bugs are pinned through the scutellum, a little to the right to the midline. Grasshoppers are pinned through the posterior part of the pronotum, just to the right of the midline.
Beetles should be pinned through the right elytron, about halfway between the two ends of the body; the pin should go through the metathorax and emerge through the metasternum so as not to damage the base of the legs. The easiest way to pin an insect is to hold it between the thumb and fore finger of one hand and insert the pin with the other. All specimens should be mounted at a uniform height on the pin, leaving about 2.5 cm free above the insect surface.
Mounting Small Insects:
ADVERTISEMENTS:
Insects too small to pin may be mounted on a card point or on a “minuten” pin. The points are elongated triangular pieces of light cardboard or celluloid, about 8 or 10 mm long and 3 or 4 mm wide at the base; the point is pinned through the base and the insect is glued to the tip of the point.
Putting an insect on a point is a very simple process. The point is put on the pin and the upper side of the tip of the point is touched to the glue and then touched to the insect.
Spreading Insects:
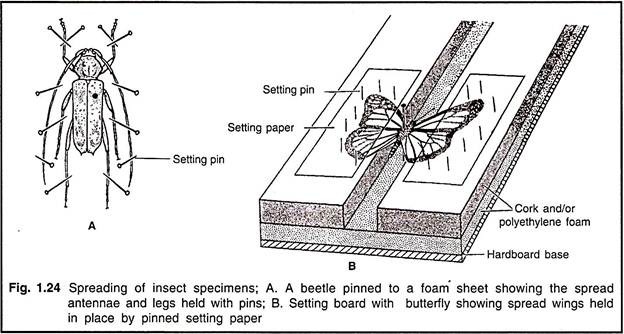
An insect that is to be a part of a collection is pinned and then spread on a spreading board with the dorsal side up (Fig. 1.24). There are certain standard positions for the wings of a spread insect. In the case of butterflies and moths, and mayflies, the rear margins of the front wings should be straight across, at the right angles to the body; and the hind wings should be far enough forward that there is no large gap at the side between the front and hind wings.
With grasshoppers, dragonflies, damselflies and most other insects, the front margins of the hind wings should be straight across, with the front wings far enough forward that they just clear the hind wings. The front and hind wings of a butterfly or moth are always overlapped, with the front edge of the hind wing under the rear edge of the front wing; in other insects the wings are usually not overlapped.
Labelling:
The scientific value of an insect specimen depends to a large extent on the information regarding the date and locality of its capture, and to a lesser extent on such additional information as the name of the collector and the habitat or food plant on which the specimen was collected. An insect collector should always label his specimens with date and locality; this is the minimum amount of data for a specimen; additional data are desirable, but optional.
Pinned insects should be kept in boxes having a soft bottom that will permit easy piercing. Large collections in institutions are frequently kept in cabinets containing drawers similar in construction to Schmitt boxes.
All insect collections are subject to attack by dermestid beetles, ants and other museum pests, and if the collection is to last any length of time, certain precautions must be taken to protect it from these pests. Various materials may be used for this purpose, but one material commonly used is naphthalene (in flake or ball form).
ADVERTISEMENTS:
Naphthalene flakes can be put into a small cardboard pillbox that is firmly attached to the bottom of the insect box, usually in one corner, and has a few pin holes in it. Paradichlorobenzene can also be used, but it volatilizes more rapidly than naphthalene and must be renewed at more frequent intervals.
Preservation of Insects in Fluids:
Some insects cannot be pinned and must be preserved in fluids. All soft-bodied adult insects, such as mayflies, stoneflies, caddisflies, aphids, most insect nymphs, all larvae, myriapods, crustaceans and arachnids should be preserved in fluid. Many minute insects, such as springtails, lice, fleas and minute flies are usually preserved in fluid until they are mounted on microscope slides.
Several chemical solutions may be used but the most important one is X.A. mixture- Xylene, one part and ethyl alcohol (95%) one part. Larvae killed with this mixture should be transferred to 75 per cent alcohol after 24 hours. This killing agent is likely to remove the bright colours of larvae, especially greens, yellows and reds.
Identification:
Identification services are provided by the Zoological Survey of India, Calcutta; Division of Entomology, Indian Agricultural Research Institute, New Delhi; Indian Forest Research Institute, Dehradun; the British Museum, Queens Gate, London, U.K., and many other national and semi-official organizations in various countries throughout the world. Before sending the specimens by post they are pinned and packed in strong and light boxes specially made for the purpose.