The following points highlight the top three haematological experiments for counting blood cells under microscope.
Experiment # 1. Estimation of Haemoglobin (HB%) (Sahil Method):
Principle:
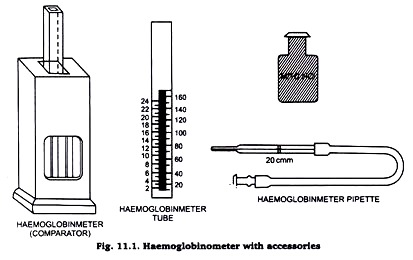
The haemoglobin per cent in vertebrate blood is estimated by acid-haematin method. Haemoglobin is converted to brown acid haematin with the addition of N/10 (0.1 N) hydrochloric acid. The brown colour is compared with standard brown glass plate (Fig. 11.1).
Requirements:
ADVERTISEMENTS:
1. Haemoglobinameter tube. It is square graduated tube marked on both the sides in ascending order. The marks on the left side show g of Hb per 100 ml and on the right side indicate percentage.
2. Haemoglobincomperator. A mounted standard brown glass plate.
3. Haemoglobin pipette. A special slender pipette with a single mark 20 c mm.
ADVERTISEMENTS:
4. Vertebrate blood.
5. Decinormal (N/10) HCL solution. (1.2 ml concentrated HCL is diluted to 100 ml with distilled water.)
Procedure:
1. The haemoglobinometer tube is filled to the lowest mark (20) with N/10 HCL.
ADVERTISEMENTS:
2. Draw blood up to 120 c mm of the pipette from finger prick (p. 533) or from some other vertebrate. Wipe off the blood on outside the pipette with gauge or tissue paper.
3. Empty the pipette in the tube by touching the point of the pipette to the bottom of the tube and gently blowing off the blood without causing bubble. Rinse the pipette at least three times by drawing in and discharging the blood-acid mixture.
4. Withdraw the pipette half way up the tube and rinse the outside of the pipette with a few drops of the acid.
5. Mix the acid-haematin solution with a glass rod and keep the tube in the comparator for at least 10 minutes. The brown colour of acid-haematin develops.
6. The brown solution is diluted drop-wise with distilled water and stirred with a stirrer until the colour matches with the standard brown glass plate in the comparater.
7. The matching of colour should be down only against natural light.
Reading:
The mark tallying with the upper level of diluted acid-haematin indicates the level of haemoglobin and is expressed in terms g per 100 ml blood.
Experiment # 2. Count of Erythrocytes (Red Blood Carpuscles: R.B.C.):
Total count:
ADVERTISEMENTS:
Total count of blood cells is usually done with a Thoma-Ziesshaemocytometer.
R.B.C. pipette:
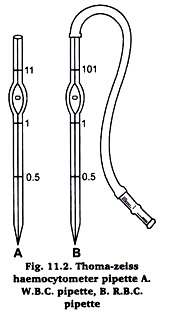
It is graduated to dilute blood 1 in 100 or 1 in 200. On the stem of the pipette is marked 0.5 and 1 and 101 just above the bulb. The bulb of the pipette has a column of 100 units and the stem a volume of 1 unit (Fig. 11.2).
Counting slide:
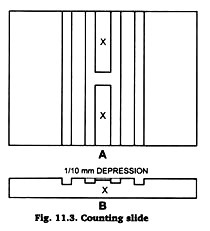
The counting chamber in the slide is of known capacity (Fig. 11.3-11.5). A special thick cover slip is used at the time of counting. The counting chamber measures 1 x 1 mm. It is divided into 5×5 = 25 small squares. Each small square measures ![]() x
x ![]() mm. It is again divided into 4×5 = 16 smallest squares (Fig. 53.4). Each smallest square measures
mm. It is again divided into 4×5 = 16 smallest squares (Fig. 53.4). Each smallest square measures ![]() x
x ![]() mm and the area is
mm and the area is ![]() sq. mm. The depth of the squares is
sq. mm. The depth of the squares is ![]() mm. Therefore the volume of one smallest square is
mm. Therefore the volume of one smallest square is ![]() x
x ![]() =
= ![]() c mm (cubic millimetre).
c mm (cubic millimetre).
Diluting fluid:
The most commonly used diluting fluid for routine classwork is formal-citrate solution. It is a solution of 1% formalin in 31.3 g/litre trisodium citrate.
Technique:
Prick the finger-tip with a needle. Wipe out first drop of blood. Suck the free flowing blood from the prick in the R.B.C. pipette held horizontally, to 0.5 mark. Wipe the tip of the pipette clean, dip vertically into the blood diluting fluid and gently suck the fluid up to mark 101 (Fig. 11.2B).
Close the tip of the pipette with finger and mix the content thoroughly with a twisting motion. The capillary of the pipette is occupied by the dilute ever, after mixing and the dilution is really 0.5 in 100, i. e., 1 in 200.
The cover slip is centrally placed over the bar of the counting chamber. After 1/3rd of the thoroughly mixed diluted blood is rejected by blowing it out of the pipette. Hold the pipette at 45° angle with the slide touching the space between the cover slip and the slab. The fluid runs under the cover slip by capillary action. Allow the flow till the space between the grooves of the slide under the cover slip is just filled.
If air bubbles are trapped under the cover slip remove those by moving the cover slip to the edge of the slab and thereby allow the air to escape. Wait for 3 minutes and check for even distribution of blood cells under the low power of a microscope. In case of eneven distribution of blood cells reject the preparation and make a fresh one. Counting is done under high power of a microscope.
Calculation:
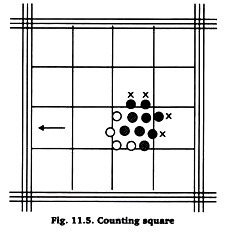
For calculation of total number of red blood corpuscles in 1 c mm (cubic millimetrel, all the carpuscles in 5 small squares, i. e., 80 smallest squares are counted.
The corpuscles touching the left and lower line of the square and those inside the square are taken into account. Those touching the right and upper line of the square are excluded (Fig. 51.5).
Volume of one smallest square = ![]() c mm.
c mm.
Volume of 80 smallest squares = 80 x ![]() =
= ![]() c mm.
c mm.
If the number of cells in ![]() c mm is X, the number of cells in 1 c mm = X × 50.
c mm is X, the number of cells in 1 c mm = X × 50.
The dilution of blood is 1 in 200.
The number of cells in 1 c mm undiluted blood = X × 50 x 200.
N.B. As a rule the total number of R.B.C. in 1 c mm undiluted blood may be obtained by adding 4 zeros to the number present in 80 smallest squares.
Experiment # 3. Count of Leucocytes (White Blood Corpuscles: W.B.C.):
Total and Differential Counting:
The counting of number of white blood corpuscles (W.B.C.) is expressed in two ways. Counting of total number of cells as total count (TC), and the count of different kinds of leucocytes separately as differential count (DC).
The five types of W.B.C. are:
(a) Polymorphonuclear neutrophils,
(b) Polymorphonucleareosinophils,
(c) Polymorphonuclear basophils,
(d) Monocytes and
(e) Lymphocytes.
A. Total Count:
Equipment’s and technique:
The equipment’s used for leucocyte count are same except the markings on pipette which allow a dilution of blood 1 in 20. The mark above the bulb is 11 in a W.B.C. pipette (Fig. 51.2A). The procedure adopted in leucocyte count is same as in R.B.C. count. Blood is down up to 0.5 mark, and W.B.C. diluting fluid up to 11 mark.
Diluting fluid:
A 3% acetic acid solution in distilled water.
Calculation:
The area of the counting chamber is 1 sq mm and the volume is 1 sq. mm x 1/10 mm. The W.B.C. in all the small squares are counted.
If the number of cells in 1/10 c mm is X, the number in 1 c mm = X × 10. The dilution of blood is 1 in 20.
The total number of W.B.C. in 1 c mm undiluted blood = X × 10 x 20 or X × 200.
B. Differential Count:
The differentiation of white blood corpuscles is baded on their size and shape of nucleus and differential staining of nucleus and cytoplasm with Giemsa or Leishman stain.
Technique:
Prepare blood film following standard technique and stain with ciemsa or Leishman stain.
Observation:
Count 100 or 200 W.B.C. noting the type on a sheet of paper and calculate the percentage.
Calculation:
The percentage of different types of W.B.C. is calculated with the formula
The normal value of various W.B.C. (type):
4. Cell count and viability assay of protozoans:
Trypan blue (diamine blue, Niagara blue or vital blue) is a toluidine derived stain with affinity for tissues and dead cells. Its name stems from its ability to kill trypanosomes the unicellular protozoa parasites that cause changes disease in America and sleeping sickness in Africa.
This stain is the most commonly used vital-stain in the world. Vital stains are used to differentiate dead from live cells. In the case of trypan blue, it is normally excluded from live cells with intact cell membranes. Dead or apoptotic cells membranes however, allow the stain to permeate the cells and stain them blue. Trypan blue is not necessary for cell counts but is essential for viability counts.
In this procedure a peripheral blood mononuclear cell (PBMC) suspension corresponding to a concentration normally achieved with ficoll preps from blood (see ficoll prep protocol) is mixed with 0.4% trypan blue solution.
The stained cells are immediately counted under microscopic observation using a Neubauerhaemocytometer. Although the original protocol suggests using equal volumes of cells suspension and 0.4% Trypan blue solution, this ratio is best optimized in each lab as variability in reagents and room temperature may indicate so.
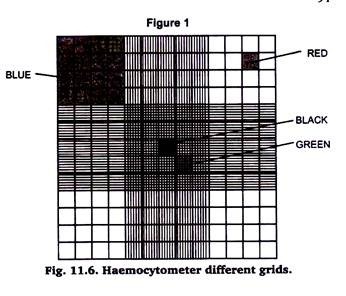
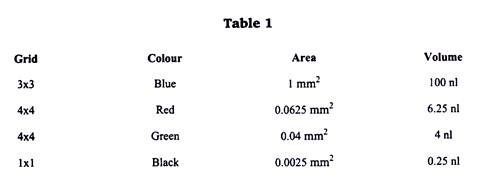
In any case, make a mental note of the dilution factor involved. Neubauerhaemocytometers are a specialized type of microscope slide that has had a grid of precise dimensions lesser etched unto it. The haemocytometer allows an exact volume of cells to be loaded into one or two of its chambers. The grid is composed of nine 1 mm2 squares (shown in blue on the image below).
Each of the four 1 mm2 squares on each of the corners is in turn divided into sixteen 0.625 mm2 square (shown in red). However, the central 1 mm2 square is divided into a much finer grid of 25 0.04 mm2 squares (shown in green) which in turn are composed of 9 squares of 0.0025 mm2 (shown in black).
As the chamber is only 0.1 mm high, each one of these squares has a fixed volume of the cell suspension (see table 1), which allows us to estimate cell concentration based on direct cell counts.
Procedure:
1. Please the 25x microscope objective in the optical path and turn illumination sourseon.
2. Estimate and record the total volume of the cell suspension from which you will take on a aliquot to count the cells.
3. Add 90 µL 0.4% Trypan blue to a well of a 96 multiwall plate or to a 0.2 mL PCR tube.
4. Max your cell suspension carefully to homogenize its contents and free cell clusters without killing the cells (finger tapping or flicking of the tube is the norm).
5. Add 10 µL of your cell suspension to the well previously loaded with the trypan blue.
6. Mix the cell suspension and trypan blue by repeat pipetting (5 to 10 times).
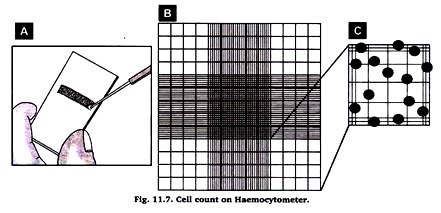
7. Take 10 µL of this trypan blue stained cell suspension and loaded unto both chambers of your haemocytometer (see figure 11.7A).
8. Place the haemocytometer on the microscope stage and let the cells stain for 1 minute before you start counting.
9. For PBMC obtained from whole blood samples through ficoll preps we normally count blue (dead) and white (live) cells from the four large corners (i. e., four blue squares such as that one shown in figure 11.7A).
10. Keep a separate count for live and for dead cells.
11. For PBMC obtained from whole samples we typically find between 20 and 50 cells (both dead and alive) per each one of these four BLUE squares, which gives us a grand total of between 80 and 200 cells for the four blue squares on the haemocytometer.
12. Cells that are found to lie halfway across the outermost boundary of each square (shown in red on figure B and C) should not be considered for the cell counts.
13. When the cell suspension has more cells than those previously mentioned (more than 50 per blue quadrant), the central quadrant should be used to better estimate their true concentration.
14. Calculate your results.
Calculations:
To calculate cell concentration present in the original cell suspension (the one from which you took the aliquot that was actually used for counting) when the four blue 4×4 corner squares of the haemocytometer are used for counting:
Concentration = Total live count ÷ 4x dilution factor x 10,000.
To calculate cell concentration present in the original cell suspension when the central 5×5 square (same size as 4×4 blue square actually) of the haemocytometer is used for counting:
(Note: This is normally used when cell count given by previous calculation is higher than 200 cells total):
Concentration = Total live count ÷ 100 x dilution factor x 10,000.
To calculate cell concentration present in the original cell suspension when only 5 green squares belonging to the central 5×5 square is used for counting:
(Note: This is normally used as an alternative to the previous calculation to speed things up risking accuracy of counts):
Concentration = Total live count ÷ 20 x dilution factor x 10,000.
To calculate total cells present in the original cell suspension from which your aliquot was taken:
Total calls = cell concentration x total volume present in original sample (the one we recorded at the very beginning).
To calculate cell viability (expressed as a per-cent):
Total number of live cells (white) ÷ Total number of cells (both dead and alive) x 100
Example:
The cell count, concentration and viability is to be assessed on a 3 mL primary lymphocyte culture. Cells are re-suspended and cell clusters gently broken up to homogenize suspension. Using a P10 micropipette 10 µL are retrieved from the cell suspension and placed in a PCR tube previously-loaded with 90 µ L of are retrieved from the cell suspension and placed in a PCR tube previously loaded with 90 µ L of 0.4% trypan blue (thus generating a 1:10 dilution of our cells).
Cells are stained for one minute and then 10 µ L are loaded up to the Neubauer slide. The cells present in the four outer 4×4 (blue) the cells present in each of the 4 outer blue squares are summed up. Thus:
Cell concentration = (455/4) x10x 10x 104 =-11’375,000cells/mL
Total cells present in my primary culture = 11375,000 x 3 = 34125,000
Cell viability = 455 ÷ 490 x 100 = 928%