In this article we will discuss about:- 1. Principle of Langendorff Heart System 2. Langendorff System-Technical Information 3. Preparation 4. Applications.
In 1895, Langendorff described studies on isolated surviving mammalian hearts, using mainly cats as donors. Since then, the method has been improved and is now used for studies on guinea-pig, rabbit or rat hearts.
Principle of Langendorff Heart System:
The principle behind the method involves the cannulation of the aorta above the sinus of Valsalva, and subjecting the heart to retrograde perfusion. The aortic valve is forced shut by the pressure of the perfusate, which then enters the coronary system, eventually leaving the heart via the cut ends of the pulmonary arteries. Throughout this procedure, the chamber of the heart remains essentially empty. By adjusting the perfusion equipment, the investigator can choose to perfuse either in a constant flow or in a constant pressure mode.
Langendorff System-Technical Information:
Both, constant flow and constant pressure systems, need a reservoir in which the buffer is aerated with carbogen (95% CO2 and 5% O2), and the pH can be checked and adjusted to 7.45-7.50, by modulating the gas output thermostated heat exchanger in which the buffer is warmed up to 37°C, and a thermostated heart chamber in which the buffer is delivered to the heart.
ADVERTISEMENTS:
The exchanger and the chamber are double jacket glassware connected to a thermostated water pump. A roller pump is also required for both systems. In the constant pressure system, it keeps the level of the buffer in the reservoir at 108 mm water column (about 80 mmHg); in the constant flow system, it drives the buffer from the reservoir into the heat exchanger at a speed sufficient to give the desired constant flow.
Electronic transducers are necessary for monitoring heart function and controlling and regulating the functionality of the Langendorff system. The most commonly used transducer is the pressure transducer connected to an isovolumic balloon inserted into the left ventricle. The balloon is inflated to a maximum pressure of 10 mmHg and gives data about the heart rate and the developed pressure. From these raw data, other functional parameters can be calculated (e.g., rate pressure product, dp/dt).
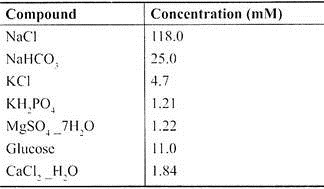
Usually Tyrode, Locke or Krebs-Henseleit buffers are used. They have to be filtered, because even the purest chemicals may contain insoluble particles which will obstruct the small coronary vessels. We use a modified Krebs-Henseleit buffer bubbled with carbogen, with a pH between 7.4 and 7.5.
Modified Krebs-Henseleit Buffer:
The perfusion buffer we normally use is the modified Krebs-Henseleit buffer.
Preparation of Hearts for Perfusion:
Anesthesia and Anticoagulation:
All animals are weighed prior to experimentation. Heparin 100 IU, is administered via intraperitoneal (i.p.) injection for prophylaxis against the formation of thrombus within the coronary vasculature or ventricular chambers. After 5 min (time allowed for activated partial thromboplastin time (APTT), to increase), the animals should be killed, either by an anesthetic overdose (e.g., sodium pentobarbital, 60 mg/kg i.p.) or by rapid cervical dislocation. The animal is then transferred to a dissection block and the limbs secured with adhesive tape.
Dissection:
ADVERTISEMENTS:
A skin incision is performed at the xyphoid sternum and continued to the lateral ends of the left and right costal margins. The incision is then continued through the ribs at the left and right anterior axillary lines to create a clam-shell thoracotomy. The anterior chest wall is deflected upwards providing an optimal operating field.
The heart is removed by transecting the descending aorta and inferior vena cava, followed by the ascending aorta and superior vena cava and transferred to a dissection dish containing ice cold Krebs-Henseleit buffer. At this stage of the experiment, the priority is to transfer the heart into the cold buffer as quickly as possible, to avoid any detrimental effects of hypoxia.
Cannulation of the Aorta and Transfer of the Heart to the Langendorff Apparatus:
The excess of extra cardiac tissues such as lungs, vessels and thymus is trimmed off and the ascending aorta is exposed and cannulated with a primed 21G stainless steel murine cannula under Krebs solution, to avoid air embolisation of the coronaries.
The cannula is inserted into the aorta by manoeuvring the heart with as little trauma as possible. This is achieved using two small forceps, which hold the atrial appendages. It should be noted that care should be taken not to insert the cannula too deep into the aorta to avoid forcing the aortic valve open and reaching into the left ventricle.
This situation is not redeemable by rapid withdrawal of the cannula as the aortic valve is invariable irreversibly damaged, resulting in a reduced coronary perfusion (buffer will be lost via left ventricle) and the heart will not perform as expected. The opposite situation, i.e., not inserting the cannula deep enough, may have the same result; if the cannula is tightly secured above the aortic branches (e.g. brachiocephalic trunk), some buffer will be lost through them.
Consequently, the perfusion flow entering the coronary system will be diminished. Therefore, it is essential that insertion of the cannula deeper than the point where the aorta leaves the base of the heart should be avoided. The aorta is secured to the cannula using a 5-0 suture, and transferred to the Langendorff perfusion apparatus.
The buffer should run slowly through the system prior to mounting the cannula, so that the heart will receive nutrients and oxygen as soon as it is placed in the system. Once securely attached to the perfusion apparatus, retrograde perfusion at a constant pressure or constant flow must be started. The time taken from the moment of the opening of the thorax until the heart is mounted and perfused on the Langendorff system has to be under 3 min, in order to avoid the potential effect of ischemic preconditioning due to delayed perfusion.
Applications of Langendorff Heart System:
Positive Inotropic Effects:
ADVERTISEMENTS:
While negative inotropic substances can be tested in a heart beating with normal force, the evaluation of a positive inotropic compound usually requires that cardiac force is first reduced. Acute experimental heart failure can be induced by an overdose of barbiturates, such as sodium thiopental, or calcium antagonists. This kind of cardiac failure can be reversed by alpha-sympathomimetic drugs, cardiac glycosides, or increased Ca+2 concentration. In this way, the potential alpha-sympathomimetic activity of a new drug can be measured, using isoproterenol as standard.
After thiopental-Na treatment, left ventricular pressure (LVP) and dp/dt max decreases considerably, whereas coronary flow is slightly enhanced. Alpha-sympathomimetic drugs restore LVP and dp/dt max and keep coronary blood flow elevated. Cardiac glycosides increase LVP and dp/dt max and leave coronary flow unchanged.
Negative Inotropic Effects:
The effects of a sympathomimetic drug such as isoproterenol at doses of 0.05 to 0.2 µg that increases contractile force as well as heart frequency are registered. After the injection of a beta-blocker, the effects of isoproterenol are attenuated. The effects of a potential beta- blocking agent can be tested by comparing the isoproterenol inhibition versus a standard, such as propranolol (0.1 mg).
Coronary Vessel Dilating Effect:
The Langendorff heart has been extensively used for assessing the coronary dilating activity of drugs.
Calcium-Antagonism:
In order to demonstrate the effect of calcium-antagonists, 1 to 5 mg BaCI2 are injected, which induce a pronounced spasm of the coronary arteries, thereby reducing the coronary flow. Five minutes later, the test drug is injected. Active compounds have a relaxing effect on the coronary arteries indicated by an increase of coronary flow.
After this effect has weaned, BaCl2, is injected again and the test drug or a standard drug, e.g., nifedipine, is tested. The increase of coronary flow is expressed as a percentage of flow during the BaCI2 spasm and compared with the effect of the standard. Using various doses, dose-response curves can be established.
Effect on Potassium Outflow Induced by Cardiac Glycosides:
In this method, by using the Langendorff heart contractile force, coronary flow, and the potassium content in the coronary outflow can be determined by flame photometry. Increase in potassium outflow correlates well with the positive inotropic effect.
Gradual Determination of Hypoxic Damage:
The enzymes, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), α-hydroxy- butyrate dehydrogenase (α-HBDH), and glutamic-oxalacetic transaminase (GOT), in the effluent of a Guinea pig heart preparation under varying degrees of hypoxia, can be measured. Potassium content and oxygen tension in the inflowing and out-flowing solution can be determined.
Arrhythmogenic, Anti-Arrhythmic and Antifibrillatory Effects:
The Langendorff heart preparation is also used to test the influence of compounds on cardiac rhythm. For recording monophasic action potential, suction electrodes are applied on the heart. Ventricular fibrillation can be induced by simultaneous injection of digitoxin (12.5 – 25.0 µg) and aconitine (12.5 – 25.0 µg) into the perfusion fluid.
Cardiac glycosides shorten the refractory period, decrease the conduction velocity and increase heterotopic stimulus generation. Aconitine increases markedly heterotopic stimulus generation. Both compounds together, invariably induce ventricular fibrillation. Anti-arrhythmic compounds can be tested in this way. Fibrillation is inhibited, at least partially, by 10-20 µg quinidine.
Electrical Stimulation and Antifibrillatory Effect:
Ventricular fibrillation can be induced in the Langendorff preparation by reducing the glucose content of the perfusion medium to 0.25 g/1000 ml and the KCl content to 0.12 g/1000 ml. After a perfusion period of 20 min, 10 µg epinephrine is injected into the perfusion cannula. Immediately afterwards, the heart is stimulated with a current of 40 Hz and 5 mA for 2 min. This procedure is repeated every 10 min.
Standard conditions are achieved when the fibrillation continues without further electrical stimulation. Hearts treated in this way serve as controls. Other hearts stimulated in the same way are treated with continuous infusion of the test drug or the standard via the perfusion medium. Differences in the incidence of fibrillations are calculated using the chi square test.