In this article we will discuss about the methods of field investigation for epidemic diseases seen in animals.
Epidemic refers to the unexpected increase in disease or death to a level clearly greater than normal. Thus, if ongoing monitoring programs exist, the level of disease or death may be referred to as an epidemic if it exceeds two standard deviations above the mean. In agriculture, however, the level of production often is the outcome of concern, not the presence or absence of disease.
Hence, by extrapolation of the earlier definition, a production epidemic might be said to exist when the level of production decreases by two standard deviations below the mean, or when the production drop reaches a critical level that signals a potential problem; this level may differ from area to area and from one production unit (farm) to another.
Also, it is entirely possible to have a production based epidemic in the absence of an epidemic of clinical disease. (Although disease often is a production limiter, disease may be one of the less important factors limiting production on a specific farm.)
ADVERTISEMENTS:
Veterinarians are frequently called to investigate outbreaks (i.e., epidemics) of disease or death. In general, the major objectives of such investigations are halting the progress of the disease, determining the reasons for the outbreak, instituting corrective measures, and recommending procedures to reduce the risk of future outbreaks.
Although the methods used to accomplish these objectives will vary from situation to situation, there are two general approaches, each dictated by the rate of spread of the problem (i.e., suboptimal productivity, disease, or death).
Specifically, disease and production outbreaks can be classified as being of the slowly spreading propagative type or of the rapidly spreading common source type. Thus, the first step in outbreak investigation is to note the temporal pattern of the outbreak (i.e., examine the epidemic curve) and ascertain whether it is likely a point (common source) or a propagated epidemic.
Although the difference between these outbreaks is somewhat arbitrary, an extremely rapid increase in the number of cases is suggestive of a common source epidemic (i.e., all animals exposed to the source at about the same time), whereas a slower build up is suggestive of a propagated epidemic. As mentioned, the method of investigation will be influenced by the temporal features of the outbreak.
ADVERTISEMENTS:
The features required for successful animal disease control programs have been described in a recent text and are beyond the scope of this book. The intent of this section is to present methods for elucidating the source of an epidemic that are broadly applicable in many settings.
The specific program(s) required to affect control will depend on the nature and scope of the problem as well as the existing circumstances. Schwabe presents two examples of propagative outbreak investigation (brucellosis and plague) and two of point epidemic investigation (botulism in humans and in mink).
Propagated Epidemics:
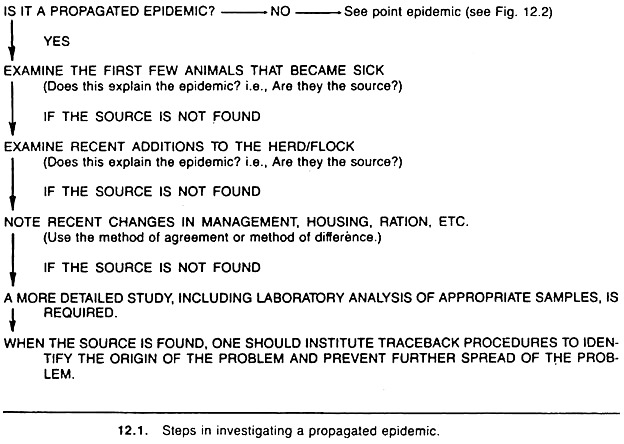
A general outline of the sequence of steps involved in the investigation of a propagated epidemic on a given premise is shown in Figure 12.1. In propagated epidemics, the agent is either spread from animal to animal by contact, or animals are initially exposed to the agent via vehicles or vectors over a protracted period of time, hence explaining its slower development relative to a common source epidemic. (It is important to emphasize that one should not assume the agent is infectious, as the frequency of chemical toxicities is likely to increase in the future.)
Because of its relatively slow rate of spread, one can usually establish a diagnosis by clinical examination of affected animals and laboratory tests. This knowledge can simplify the investigation process; however, it is not an essential step and one should not devote undue time to establishing the diagnosis, at least initially.
ADVERTISEMENTS:
Whether or not a diagnosis is established, one should attempt to identify the first few animals to become sick and note their characteristics (e.g., if they are recent purchases or if they have been in contact with other animals and/or premises).
If the disease under investigation is identified and if the first few animals to become ill are the likely source of the agent in the current outbreak, then isolation, treatment, or removal of these animals may be appropriate.
Trace back to their origin may be essential if the disease is serious, infectious, and/or if it is a disease for which government veterinarians have legislative responsibility (e.g., notifiable diseases).
If no obvious clues are provided following an examination of the first few animals to become diseased, and if the disease appears to have an infectious etiology, it is important to inquire about recent animal acquisitions; many times these animals are carriers and may not develop clinical disease.
Again, if the investigation implicates particular animals as the likely source(s) of the problem, isolation, treatment, removal, and/or trace-back of these animals may be necessary.
If no animals or other logical sources of the problem have been identified, data should be collected and analyzed on possible environmental sources of the agent, in a manner similar to that performed in the investigation of common source epidemics. If the problem is localized to one premise, note, for example, details about changes in management, husbandry, purchase of new feed, and ventilation.
In this approach, one searches for a factor common to all affected animals (i.e., method of agreement), or a factor that differs between affected and normal animals (i.e., method of difference) on that premise.
The latter methods would also be applicable if the problem involved more than one premise, thus, search for factors common to all affected farms and/or for factors whose presence differs between affected and non-affected farms using a case- control approach.
Point Epidemics:
ADVERTISEMENTS:
The general sequence to be followed when investigating a point epidemic is presented in Figure 12.2. In this regard, standardized procedures for investigating common source outbreaks have been developed.
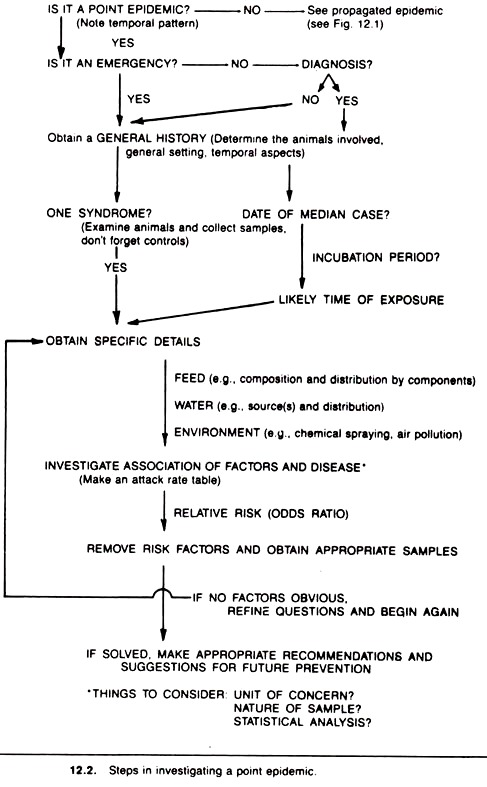
The private practitioner is usually called to investigate a point epidemic before it reaches its peak. As such, these are emergency situations and the veterinarian must act quickly to find the source and prevent the exposure of more animals. Less often, the epidemic has run its course prior to the time the veterinarian is called.
In both cases, the task is the same, and although the sequence of the investigation will vary from situation to situation, it is very important to recognize a point epidemic early in its course, focusing attention toward finding the common source, be it air, food, or water. Again, it is important to emphasize that one ought not assume an infectious agent as the cause of the disease.
In an emergency situation, little time should be spent attempting to diagnose the disease condition, unless a diagnosis is obvious. Initially, one should obtain a general history, including data on host, husbandry, geographic, and environmental factors.
Examine some of the affected animals clinically, and if appropriate at postmortem, to ascertain the main features of the syndrome and whether the outbreak is consistent with one syndrome. Clinical examination or postmortem examination can often pinpoint the nature of the disease and its likely source.
In the absence of a diagnosis, and/or if more than one syndrome is involved (the rare case), the investigation procedure may need to differ for each syndrome. In either event, adequate tissue and other samples should be taken and either submitted for immediate laboratory processing or stored for future laboratory work as the situation dictates.
This may entail sampling affected animals as well as clinically normal animals in the high risk area, or animals outside the high risk area (the latter “normals” provide necessary benchmark data in case the responsible agent proves difficult to identify).
Besides directly indicating the disease and/or its source, if a diagnosis can be made, it may assist indirectly in identifying the source and nature of the problem, because many agents have a strong association with specific sources (e.g., salmonella with poultry products and powdered milk).
In addition, and of greater utility, for many diseases the average incubation period can be used to determine the most likely time(s) of exposure. (In food-borne outbreaks of disease in humans, information on the incubation period is often used to identify the most likely meal at which exposure occurred.)
This can be done quite formally in retrospective investigations of outbreaks. To accomplish this, the midpoint of the epidemic (usually the date the 50th percentile —median —case occurred) is identified and then the average incubation period for the disease is subtracted from this to identify the most likely time and/or place of exposure.
At this point in the investigation, the investigator usually has sufficient information to formulate some theories about the reason(s) and source(s) for the outbreak. Based on this information, appropriate questions regarding the environment, food, and water are developed.
If feedstuffs were suspected one would collect data on the composition of the ration and look for associations between each ration component and disease (e.g., in a feedlot outbreak, compare the distribution of roughages and concentrates to the distribution of disease).
Usually, one or more suspect sources will be identified at this stage of the investigation. It is often a good idea to take some samples at this point. The samples may be submitted to a laboratory if a reasonably good diagnosis has been made; alternatively, they may simply be stored for possible future use. If no associations are found for air, water, or feed, the cycle should be repeated using more refined questions.
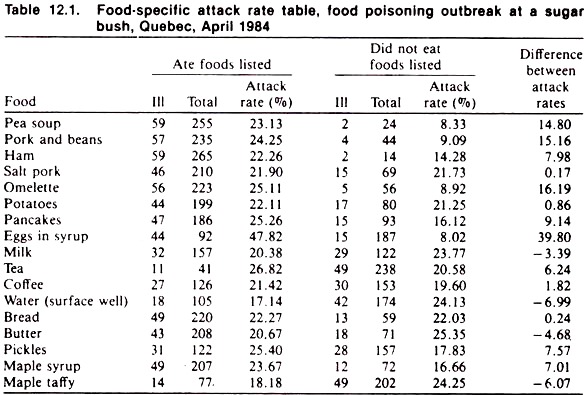
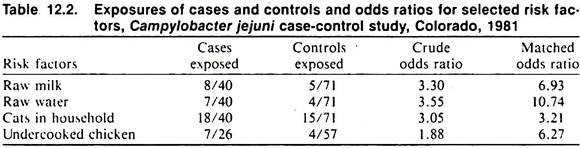
Although the investigation often is performed under less than ideal circumstances, it is advisable to record all collected data as neatly, completely, and in as orderly a manner as possible. Tabulation of data in the form of an attack rate table is useful (see Tables 12.1 and 12.2).
Attack Rate Table:
In constructing an attack rate table, one must first decide whether the individual animal or a group (e.g., a litter or pen) is the correct unit of analysis. As a guideline, if the animals are housed, fed, or watered as a group the correct unit is the group.
Initially, when the group is the unit of analysis, it will suffice to classify each group of animals as affected or not. The actual level (i.e., morbidity or mortality rate) of disease within a group may be required only for detailed studies or for final interpretations.
Second, it is important to consider whether data are available from a representative cross-section of the population at risk, or whether the data are obtained in a case-control fashion (i.e., data are obtained from some or all of the cases and from some of the unaffected population).
In the former case, the risk (rate) of disease can be directly determined for each suspect factor (Table 12.1). In the latter case, direct estimates of attack rates are not possible and one must compare the proportion of cases that were exposed to a factor to the proportion of exposed non-cases using the odds ratio (Table 12.2).
A third consideration is whether to use formal statistical tests to evaluate the probability of “chance variations” explaining the observed differences. In general, formal testing is required only in the final stages of the investigation and/or in nonemergency outbreak investigations.
Once the data are summarized and tabulated, the following guidelines are useful to determine if an item (e.g., a ration component) is the source of the agent.
If exposure to a specific source (factor) is the cause of the outbreak:
(1) There should be little or no disease in unexposed units (i.e., individuals or groups);
(2) Most of the affected units should be exposed to that item; and
(3) As a result of 1 and 2, the relative risk (or odds ratio) and/or the attributable rate should be large for exposure to that item.
A general guideline is to ignore those items to which all the population at risk is exposed, unless it is possible to refine questions about these items and hence investigate the relationship between sub-items and the disease.
For example, all cattle in a feedlot might receive silage, yet rather than ignoring this item, one might inquire about different lots of silage before proceeding. On the other hand, if there is only one source of water for all animals and the distribution of the outbreak is not uniform throughout the population, water could be considered an unlikely source of the problem.
The data in Table 12.1 relate to an outbreak of food poisoning in people who had eaten at a maple sugar bush party in Quebec, Canada. The food items served at the bush party are listed and the number of people eating and not eating each food item noted, together with the number in each of these groups that became ill.
After calculating the attack rates, the attributable rate is calculated for each food item; the food item with the largest attributable rate being the most likely source of the problem. (The relative risk or odds ratio could also have been used for this purpose.)
In this instance, the eggs in syrup were the most likely source; culturing of these yielded a coagulase-positive staphylococcus. Later, this organism was demonstrated to be of the same phage type as was found in the stool and vomitus of the ill people.
The data in Table 12.2 relate to selected risk factors for cases of Campylobacter jejuni infection (only those factors producing an elevated odds ratio are shown). Since the total exposed population was unknown, the sampling of affected and non-affected people was done in a case-control fashion Cases were obtained from hospital and laboratory records. Two controls were chosen for each case and were matched for age and sex.
One control was the nearest neighbor of the case, the other was a best friend of the case. Because of this selection procedure, disease rates by item cannot be calculated; hence odds ratios are used to measure the strength of association. Note that the odds ratios (Table 12.2) were larger when the analysis considered the matching; this would indicate that age and/or sex were related to both the risk factors and C. jejuni infection.
The risk factors themselves were interrelated and the Mantel-Haenszel procedure was used to control for these (data not shown). Two of the risk factors for infection, raw water and raw milk, are also associated with large-scale outbreaks of C. jejuni intestinal disease in man.
At this stage of the investigation, sufficient information should be available to identify and remove (control) the most likely sources. Samples of the suspect items should be collected, if they haven’t been, for future microbiologic or chemical analyses. In special circumstances, a feeding or exposure trial can be used to quickly evaluate suspect sources (e.g., using laboratory animals, or using “poor-doers” in a chronic-case pen in a feedlot or swine facility).
A final and important step is to recommend procedures to prevent recurrence of the problem. These might involve suggestions about using a different water source, different ration preparation and handling procedures, or ensuring appropriate ventilation when agitating slurry. A detailed written report outlining the investigation, its findings, and recommendations should be given to the client at the termination of the investigation.
If multiple premises are involved in an outbreak, the approach is similar to that outlined. If the temporal pattern of the outbreak suggests a point epidemic, and only one syndrome appears to be present (in the absence of a confirmed diagnosis), a formal search for the common source should commence. The unit of analysis is the premise (e.g., herd or flock).
If the outbreak appears to resemble a propagated epidemic, the investigation may be more complex. Nonetheless, examining the first premise to report problems for recent additions (of animals, feedstuffs, equipment changes, fertilizers, insecticides, etc.) should be an early task as one is looking for a single factor common to all farms.
In addition, a formal comparison of the characteristics of, and recent happenings on, affected farms to the characteristics of nearby non-affected farms using the attack rate table approach should prove useful in identifying the source of the problem. Again, although a confirmed diagnosis is helpful in directing the investigation, one should not delay the collection of appropriate data by waiting for a diagnosis to be made.
Such a delay may prove costly because the source (e.g., contaminated feed) may continue to spread and/or the source (e.g., contaminated feed or disinfectant) may be used before appropriate samples are collected. In the latter instance, it may never be possible to complete the investigation, leaving the client and the investigator with only circumstantial evidence.