In this article we will discuss about soaps and synthetic detergents.
Soaps:
Soaps are salts of long chain fatty acids. Alkaline hydrolysis of a fat or oil produces a soap and glycerol. The older method of soap production consisted of treating molten tallow (the fat of livestock) with a slight excess of alkali in large open kettles.
The mixture was heated and steam was bubbled through it. After the saponification process was completed, the soap was precipitated by the addition of sodium chloride, and then filtered and washed several times with water.
ADVERTISEMENTS:
It was then re-precipitated from the aqueous solution by the addition of more NaCl. The glycerol was recovered from the aqueous wash solution. Currently, soap is prepared by a continuous process wherein lipids are hydrolysed by water under high pressures and temperatures (700 lb/in2 and 200°C). Na2CO3 is used (as given in the following figure) instead of the more expensive NaOH to neutralize the acid.
The crude soap is used as industrial soap without further processing. Pumice or sand may be added to produce scouring soap. Other ingredients, such as perfumes and antiseptics are added to produce toilet soaps and de-odourising soaps, respectively. If air is blown through molten soap, a floating soap is produced. Such a soap is not necessarily purer than other soaps; it merely contains more air.
Ordinary soap is a mixture of the sodium salts of various fatty acids. Potassium soap is a mixture of the sodium salts of various fatty acids. Potassium soaps (soft soap) are more expensive but produce a softer lather and are more soluble. They are used in liquid soaps and shaving creams.
Cleansing Action of Soaps:
ADVERTISEMENTS:
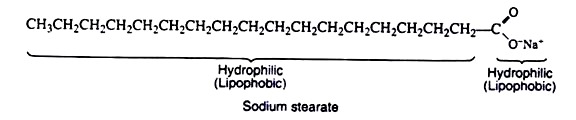
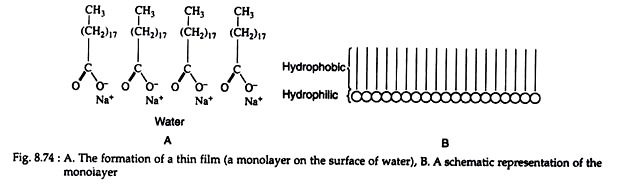
A soap molecule can be considered to be composed of a large nonpolar hydrocarbon portion (hydrophobic—repelled by water) and a carboxylate salt end (hydrophilic—water soluble), e.g. sodium stearate. When soap is added to water, the hydrophilic ends of the molecules are attracted to the water and dissolved in it, but the hydrophobic ends are repelled by the water molecules.
Consequently, a thin film (suds) forms on the surface of the water, drastically lowering its surface tension (Fig. 8.74).
When the soap solution is brought into contact with grease or oil (most dirt is held to clothes by a thin film of grease or oil), soap molecules become reoriented. The hydrophobic portions dissolve in the grease or the oil, and the hydrophilic ends remain dissolved in the aqueous phase.
ADVERTISEMENTS:
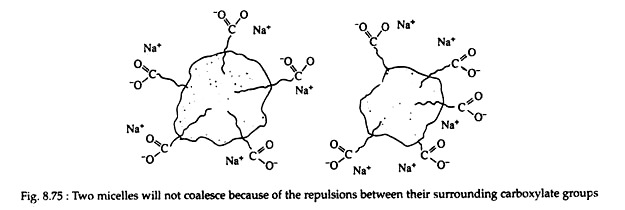
Mechanical action, such as scrubbing, causes the oil or grease to disperse into tiny droplets, and soap molecules arrange themselves around the surface of the globules. Oil or grease droplets surrounded by soap molecules are examples of micelles (a micelle is an aggregate of dipolar species.)
Because the carboxylate ends of the soap molecules project outward, the surface of each drop is negatively charged; therefore, the drops repel one another and do not coalesce (Fig. 8.75). The entire micelle becomes water soluble and is able to be washed away by a stream of water. The cleansing process involves both the lowering of the surface tension of water and emulsification.
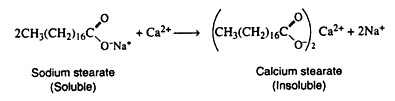
One major disadvantage involved in the use of soap results from the presence of certain metal ions in hard water. Calcium and magnesium ions form precipitate with the carboxylate ion of fatty acids. These precipitates are responsible for bathtub rings and the white insoluble curd found at the bottom of washing machines.
The various methods used for softening hard water all involve the removal of the calcium and magnesium ions. The equation representing the action of hard water upon soap is given.
Synthetic Detergents:
ADVERTISEMENTS:
The term detergent is a rather general one, used to denote any cleansing agent. Soaps would fall under such a broad definition. However, the popular use of the word generally refers to synthetic detergents also called syndets. Syndets have the desirable property of not forming precipitates with the ions of hard water. They may be classified as either anionic, cationic, or nonionic.
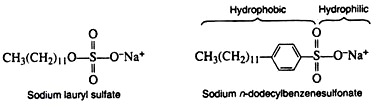
1. Anionic Detergents:
Anionic detergents are sulfates of fatty acids or sulfonate salts of hydrocarbons.
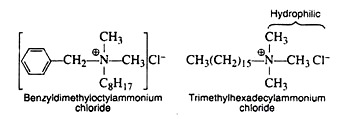
2. Cationic Detergents:
These detergents are sometimes referred to as invert soap because their water-soluble end carries a positive, rather than a negative, charge. In addition to being good cleansing agents, they process germicidal properties and are widely used in hospitals for this reason.
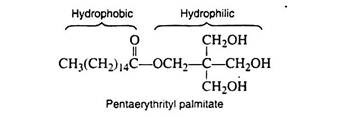
3. Nonionic Detergents:
These detergents contain polar covalent structures that provide the required water solubility. They are used extensively in dishwashing liquids and on all occasions that call for the absence of inorganic ions
4. Environmental Effects:
The large scale use of synthetic detergents created a serious disposal problem. Soaps which contain straight chain alkyl groups, can be removed from waste water through degradation by microorganisms in the soil (septic-tanks) or in sewage treatment plants. Soaps are, therefore, said to be biodegradable. Many of the synthetic detergents could not be removed in this manner.
The metabolism of microorganisms is adapted to the straight chain alkyl groups found in soaps and natural fats; they are unable to break down the highly branched analogs used in the early syndets. The synthetic detergents continued to foam and make suds, which clogged waste disposal plants, killed fish and wildlife by polluting streams, and even managed to make their way into city drinking water.
A second environmental problem caused by detergents has not been solved. In their search for more effective cleansing agents, manufacturers have added builders to their detergents.
Builders have little detergent effectiveness alone, but mainly function:
(1) To soften water,
(2) To prevent soil from re-depositing on clothes, and
(3) To maintain a proper level of alkalinity in the wash water.
Although a number of inorganic compounds have been used as builders (e.g. carbonates, bicarbonates, borates, silicates), the phosphates are the most effective. A typical detergent might contain as much as 50% phosphate. Approximately, half of the phosphate content of domestic sewage was contributed by detergents, the remainder being derived from human wastes.
Phosphate is a nutrient required for plant and animal growth. It has thus been implicated as the principal cause of eutrophication of lakes and rivers. Overabundance of plants, especially algae (algae blooms), and decreased level of oxygen.
Only certain species of fish (carp, crappie, perch, and bullhead) can live in such an environment. Moreover, the algae can produce chemicals that cause unpleasant tastes and smells in water and that, in some cases, are toxic to animals.