In this article we will discuss about the meaning and classification of carbohydrates.
Meaning of Carbohydrates:
The name carbohydrate is used to designate the large class of compounds that are ploy-hydroxy aldehydes or ketones, or substances that yield such compounds upon acid hydrolysis. Therefore, carbohydrate literally means hydrate of carbon.
Carbohydrates are most important energy providing substrates for animals. It is the major component of most plants, comprising from 60-90% of their dry mass. In contrast, animal tissue contains a comparatively small amount of carbohydrate (e.g., less than 1% in man).
General Structure:
ADVERTISEMENTS:
Carbohydrates contain only three elements-carbon, hydrogen and oxygen. The ratio of hydrogen to oxygen is typically 2: 1 in the molecule. The general structural formula is Cx(H2O)y.
Classification of Carbohydrates:
According to the number of basic sugar or saccharide units incorporated in the molecule – carbohydrates can be classified into monosaccharides, disaccharides and polysaccharides. For the most part, the mono- and disaccharides are sweet in taste, crystalline solids that are readily soluble in water. Polysaccharides are frequently tasteless, insoluble, amorphous compounds with exceedingly high molar molecules.
1. Monosaccharides:
The general names for the monosaccharides are obtained in a manner analogous to the naming of organic compounds- by the IUPAC system. The number of carbon atoms in the molecule is denoted by the appropriate prefix; the suffix ose is the generic designation for any sugar.
ADVERTISEMENTS:
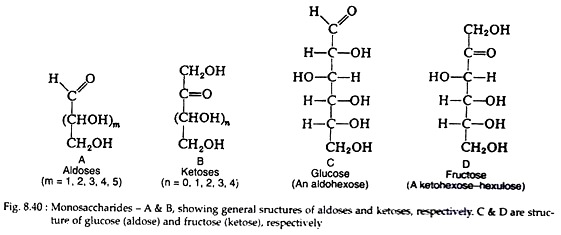
For example, the terms triose, tetrose, pentose and hexose signify 3, 4, 5 and 6 carbon monosaccharides, respectively. In addition, those monosaccharides that contain an aldehyde group are called aldoses, e.g. glucose; those containing a ketone group are ketoses, e.g. fructose. The general formula and structure of any aldose and any ketoses are described in Fig. 8.40.
Stereochemistry:
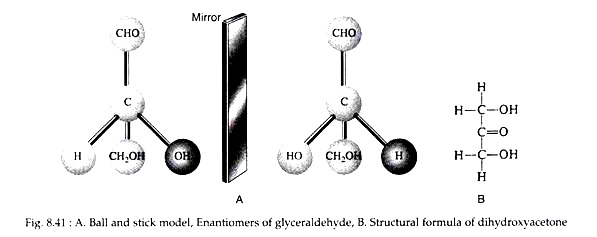
The simplest monosaccharides are the trioses, e.g., glyceraldehyde and dihydroxyacetone. Glyceraldehyde possesses an asymmetric carbon atom and, thus, may exist in two optically active forms. Dihydroxyacetone does not contain a centre of asymmetry. Figure 8.41 shows a ball and stick models of the two forms of glyceraldehydes and the structural formula of dihydroxyacetone (Fig. 8.41).
The two glyceraldehyde isomers are clearly mirror images. Except for the direction in which they rotate plane-polarized light, they have identical physical properties. One form has a specific rotation of -13.5°. The great German chemist Emil Fischer (Nobel Prize, 1902) initiated a convention of projecting the formulas on a two dimensional plane.
In this system the aldehyde group is written on the top, with the hydrogen and hydroxyl written to the right and left. (Formulas of sugars represented in this manner are referred to as Fischer projections, Fischer models or Fischer configurations).
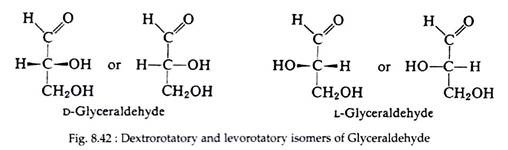
Arbitrarily, Fischer then decided that the formula of glyceraldehyde in which the hydroxyl group is positioned to the right of the asymmetric carbon atom represents the dextrorotatory isomer. He assigned the “letter d as its prefix. The levorotatory isomer, in which the -OH group is positioned to the left of the asymmetric carbon atom, was accordingly assigned the letter l as its prefix (Fig. 8.42).
The two forms of glyceraldehydes are especially important because the more complex sugars are considered to be derived from them. They serve as a reference point for designating and drawing all other monosaccharides.
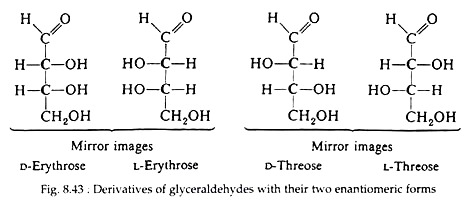
Sugars whose Fischer projections terminate in the same configuration as D-sugars; those derived from L-glyceraldehyde are designated as L-sugars. The convention is illustrated by a consideration of the four-sterioisomeric aldotetroses (Fig. 8.43).
Optical isomers that are non-super- imposable mirror images are called enantiomorphs (Greek, enantios = opposite), which is often abbreviated as enantiomers. Notice that D- and L-erythrose and D- and L-threose are pairs of enantiomers.
However, D-threose and L-threose are diastereomers of D-erythrose and L-erythrose. Thus, aside from having the same molecular formulas, the erythroses and threoses are completely different sugars, having completely different chemical and physical properties.
ADVERTISEMENTS:
The letters d and l are often misleading for the beginners. It must be emphasised that these prefixes serve only to signify the absolute configuration of the molecule. A D-sugar is one that has the same configuration about the penultimate carbon atom, as does D-glyceraldehyde. The penultimate carbon is the asymmetric carbon atom that is farthest from the aldehyde or ketone group.
The penaltimate carbon has been chosen, by convention, to be the reference carbon atom. The letters do not in any way refer to the optical rotation of the molecule. D-glyceraldehyde is dextrorotatory. It is not to be expected, however, that all compounds derived from it will have the same optical rotation, since such compounds may have some additional asymmetric carbon atoms.
In fact, D-threose and D-erythrose are both found to be levorotatory. The direction of rotation of plane-polarised light is the specific property of each optically active molecule. It is not at all dependent upon the configuration of the penultimate carbon.
The symbol plus (+) and minus (-) are used to denote the optical rotation of the monosaccharides. Plus (+) indicates a clockwise or dextrorotatory rotation and minus (-) indicates an anti-clockwise or levorotatory rotation.
The correct designations for the isomers of glyceraldehyde, erythrose, and threose that indicate both their configuration and their optical rotation are given as follows: D (+)-glyceraldehyde, L (-)-glyceraldehyde, D (-)-erythrose, L (+)-erythrose, D (-)-threose and L (+)-threose.
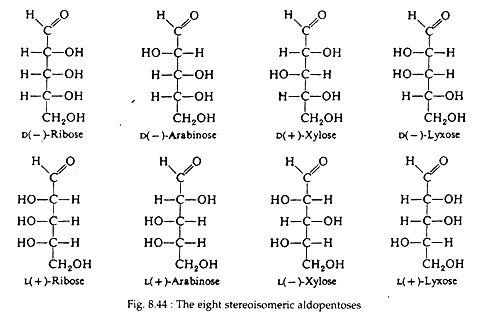
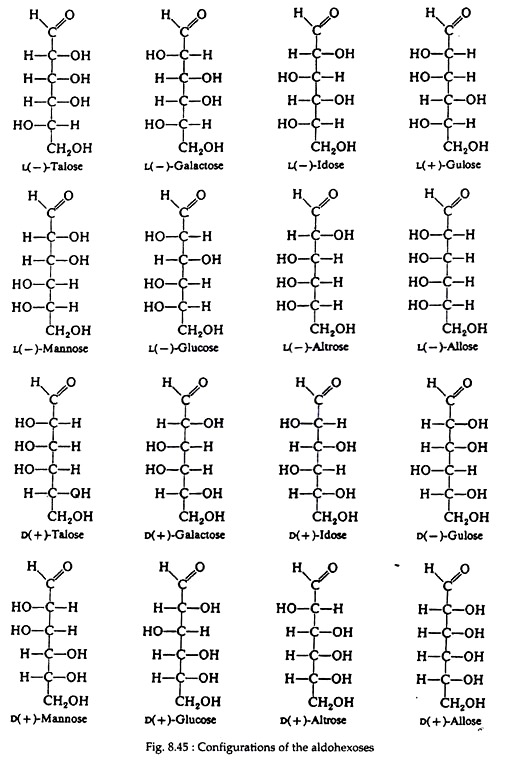
Fig. 8.44 shows all possible stereo- isomeric aldopentoses and their complete names. Notice that the aldopentoses contain three asymmetric carbon atoms and that there are four enantiomeric pairs. Aldohexoses (Fig. 8.45) contain four asymmetric carbon atoms and thus there are eight enantiomeric pairs, or sixteen isomers.
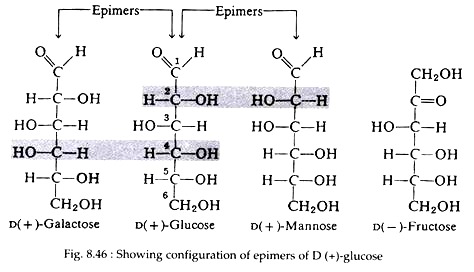
Fortunately for the students of biochemistry, only three of the sixteen isomers are commonly found in nature, D (+)-glucose, D (+)-mannose, and D (+)- galactose. None of these three stereoisomers is a mirror image of either of the others; so all three are diastereomers of each other. Furthermore, D (+)-glucose differs from D (+)-mannose and D (+)-galactose only in the configuration of one asymmetric carbon atom, carbon numbers 2 and 4, respectively.
D (+)-Mannose, and D (+)-galactose are said to be epimers of D (+)-glucose (Fig. 8.46). A pair of diastereomers that differs only in the configuration of a single carbon atom are said to be epimers. No epimeric relationship exists between D (+)-mannose and D (+)-galactose. Finally, it should be mentioned that aldohexoses are structural isomers of the only naturally occurring ketohexose, D (-)-fructose (Fig. 8.46).
Cyclic forms of sugars:
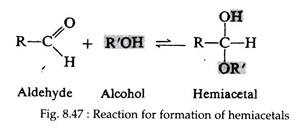
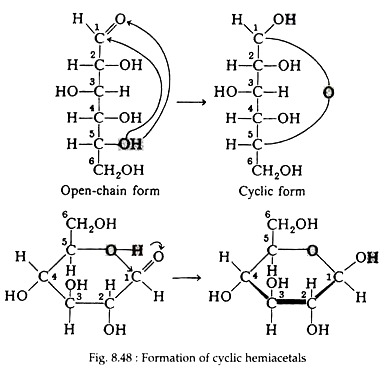
Till now sugars are considered as open- chain structures containing a carbonyl group. Studies of the physical and chemical properties of these sugars indicate that other forms predominate, both in solution and in solid state. E.g. there are two crystalline forms of D-glucose, each having distinctly specific rotations. It is known that alcohol may react with aldehyde to form unstable compounds called hemiacetals.
The reaction (Fig. 8.47) is said to be inter- molecular, since it involves reaction between two distinct molecules. Because of the geometry of the glucose molecule, however, the hydroxyl group on carbon number five can react intra-molecularly with the carbonyl group to form a stable, cyclic hemiacetal (Fig. 8.48).
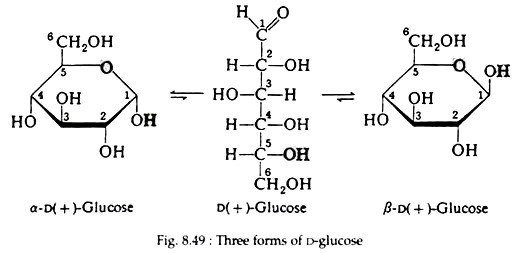
As a result of the cyclisation reaction, a six-membered ring containing five carbon atoms and one oxygen atom has been formed. In this book, the convention first suggested by an English Chemist, Sir W. N. Haworth, for representing the formulas of the cyclic forms, will be used. The molecules are drawn as planar hexagonal slabs with darkened edges toward the viewer. Ring carbon atoms and hydrogen atoms directly attached to them are not shown.
The disposition of the hydroxyl groups, positioned either above or below the plane of the ring, is sufficient to define the correct configuration of the molecule. Any group of atoms written to the right in the Fischer projection appears below the plane of the ring, and any group written to the left appears above the plane. The formulas for the three forms of D-glucose are shown in Fig. 8.49.
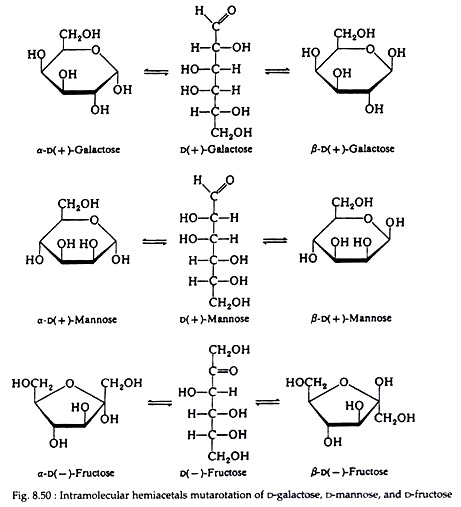
Intra-molecular hemiacetal is also present in galactose, mannose and fructose. Fig. 8.50 shows the equilibrium between the three forms of D-galactose, D-mannose and D-fructose.
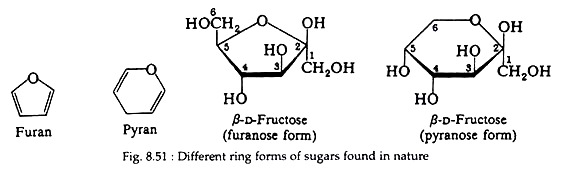
Notice that galactose and mannose, like glucose, form a six-membered cyclic structure. Fructose can also exist in this form but is most commonly found in nature, existing as a five-membered ring. Sugars with six-membered rings are called pyranoses because they are related to the heterocyclic compound pyran; those with five- membered rings are furanoses and are related to the heterocyclic compound furan (Fig. 8.51).
Some important monosaccharides:
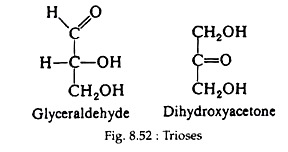
I. Trioses:
Both glyceraldehyde and dihydroxy-acetone (Fig. 8.52) are found in animal and plant cells and play important roles in carbohydrate metabolism.
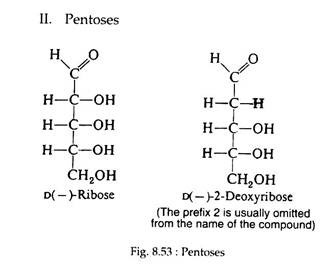
II. Pentoses:
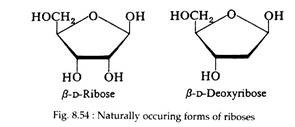
Ribose and deoxyribose (Fig. 8.53 and 8.54) are commonly found in nature. In the cyclic form they possess furanose structures:
Both sugars are found in the furanose form in the nucleic acids of all living cells. Ribose is also an intermediate in the pathway of carbohydrate metabolism and is a constituent of several of the coenzymes.
III. Hexoses:
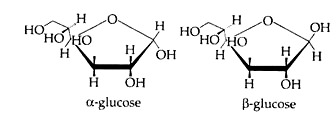
(a) Glucose:
The most common monosaccharide is a hexose – glucose, whose empirical formula is C6H12O6. There are two forms of glucose – α and β (Cyclic forms are given below). Two molecules of α form condense to form maltose and much glucose condensed to form starch. Whereas two molecules of β glucose condense to form cellulose via a transition product cellobiose.
Glucose is commonly found in fruits, especially in ripe grape, and for this reason it is often referred to as grape sugar. It is also known as dextrose, a name that derives from the fact that the predominant form of the glucose is dextrorotatory.
The majority of carbohydrates taken in by the body are eventually converted to glucose in a series of metabolic pathways. It is the circulating carbohydrate of animals. The blood contains about 0.08% glucose in man. Commercially glucose is made by the hydrolysis of starch.
(b) Galactose:
Galactose is a kind of hexose isomer, which is obtained by hydrolysis of lactose, a disaccharide composed of a glucose unit and a galactose unit. It does not occur in nature in the Free State. Galactose is needed by the human body for the synthesis of lactose in the mammary glands. Moreover, it is an important constituent of the glycolipids that occur in the brain and in the myelin sheath of nerves.
(c) Fructose:
It is the only naturally occurring keto-hexose, and it occurs predominantly in the furanose form (as in sucrose, an inulin). This sugar is also referred to as levulose because it has an optical rotation that is strongly levorotatory. It is the sweetest sugar, and it is found, together with glucose and sucrose, in sweet fruits and honey.
2. Disaccharides:
The monosaccharide can form condensation products by means of glycosidic bonds between particular atoms in adjacent sugars. The products are hence called glycoside. The general term glycoside is used to refer to the acetal that is formed when any carbohydrate reacts with a hydroxy compound. Specific compound may be named according to the sugars from which they are derived: hence glucoside from glucose, galactoside from galactose, etc.
The carbon-oxygen-carbon bond that joins the two components of the acetal is called glycosidic bonds. Such bonds may also join monosaccharides to sterols and other chemicals. Besides, monosaccharides also forms bond with other molecules via Nitrogen Bridge, e.g., ribose bonds to nitrogen bases.
Some important disaccharides:
I. Sucrose:
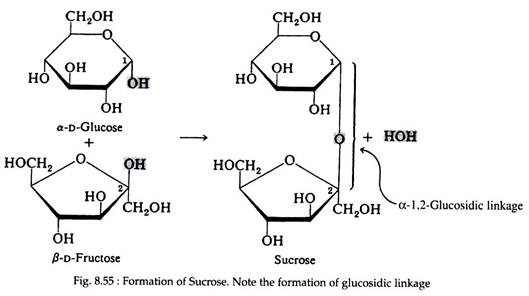
Sucrose is known as beet sugar, cane sugar, and table sugar or simply as sugar. It is probably the largest selling pure organic compound in the world. A molecule of sucrose may be envisioned from the combination of one molecule of α-D-glucopyranose and one molecule of β-D-fructofuranose; a molecule of water is eliminated in the process.
The glucosidic bond in the sucrose molecule is unique – it involves the hydroxyl group on carbon number one of α-D-glucose and the hydroxyl group on carbon numbers two of β-D-fructose. By convention, sugars are read from left to right. This connecting link is, therefore, a α-l, 2-glucosidic linkage (Fig. 8.55).
This bonding bestows certain properties upon sucrose that are quite different from those of other disaccharides. One of the properties, is that sucrose molecule remains intact and cannot uncyclise to form an open chain structure. Sucrose, therefore, does not undergo reactions that are typical of aldehydes and ketones, and it is said to be a non-reducing sugar.
Human body is unable to utilize sucrose or any other disaccharide directly because such molecules are too large to pass through the cell membranes. Therefore, disaccharides must first be broken down by hydrolysis into its constituent monosaccharide units.
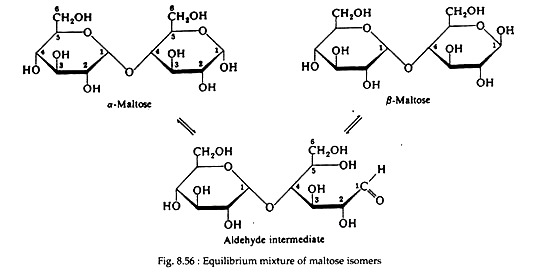
II. Maltose:
It is a disaccharide of two glucose molecules. The glucose units in maltose are joined in a head-to-tail fashion through an alpha linkage from carbon number one of one glucose molecule to carbon number four of the second glucose molecule (i.e., a α-1, 4- glucosidic linkage).
Maltose is reducing sugar and does not occur in Free State in nature. It can be found from the enzymatic hydrolysis of the starch. In the manufacture of beer, maltose is liberated by the action of malt (germinating barley) on starch, and for this reason it is often referred to as malt sugar.
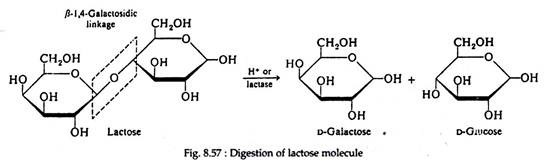
III. Lactose:
It is a disaccharide composed of one molecule of D-galactose and one molecule of D-glucose joined by a β-1, 4-galactosidic bond.
It is a reducing sugar and exclusively associated with animal kingdom. It is known as milk sugar because it occurs to the extent of about 5% in mammalian milk.
3. Oligosaccharides:
Sometimes another group is used to classify carbohydrates. This is called oligosaccharide (includes polymers from 3 to 10 sugar units in their molecules). This can be formed during the process of hydrolysis of polysaccharides.
4. Polysaccharides:
The molecule where ten or more sugar monomers are linked together is called polysaccharide. Polysaccharides are non-reducing carbohydrates, and are not sweet in taste.
These are of two types:
(a) Homo-polymers/Homiglycan polysaccharides:
All the monomers are the same in the homo-polymer/homiglycan polysaccharides, e.g. cellulose, which is a structural polysaccharide, synthesised from β glucose units. Amylose is also called as polyglucose.
(b) Hetero-polymer/Heteroglycan polysaccharides:
Here all the monomers are different. E.g., sugar acids, amino sugars etc. They are very common in nature (gums, pectin, and hyaluronic acid). Hyaluronic acid is a component of mammalian connective tissue, vitreous humour of eye and in the movable joints as a part of the synovial fluid. It is also associated with collagen.
Some important polysaccharides:
I. Starch:
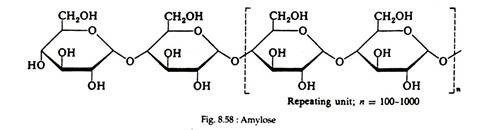
Starch is a heteroglycan made up of two polymers, amylopectin and amylose. Amylose is a straight-chain polysaccharide composed of d-glucose units joined by a α-1, 4-glucosidic linkage, as in maltose. Thus, amylose might be thought of as either polymaltose or polyglucose (Fig. 8.58).
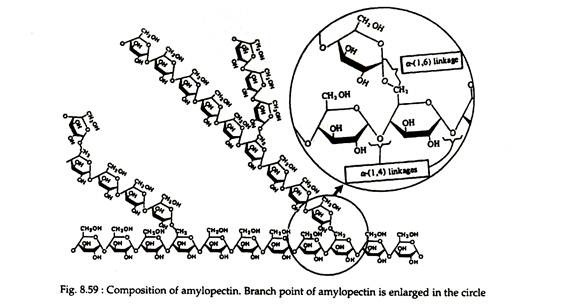
Amylopectin is a branched-chain polysaccharide composed of glucose units that are linked primarily by a α-1, 4-glucosidic linkage, but have occasionally α-1, 6-glucosidic linkages, which are responsible for branching (Fig. 8.59).
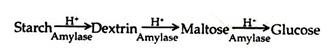
The complete hydrolysis of starch occurs in three successive stages – dextrin, maltose and then to glucose. Heating in the presence of dilute acid can hydrolyse starch. In human body several enzymes known collectively as amylase degrade it sequentially:
II. Glycogen:
It is similar in structure to amylopectin, but it is more highly branched. Glycogen can be broken down into its D-glucose sub-units by acid hydrolysis or by means of amylase as in case of starch. In animals, the enzyme phosphorylase catalyzes the break-down of glycogen into phosphate esters of glucose. It is often called as animal starch, and is the reserve carbohydrate of animals. All animal cells store glycogen for emergency purpose, but it is abundant in liver, 4-8%, and in muscle cells 0.5-1%.
III. Cellulose:
Cellulose is fibrous carbohydrate found in all plants and serves as structural component of the plant’s cell wall. Partial hydrolysis of cellulose yields only cellobiose. Its molecule is composed of D-glucose units joined by β-1, 4-galactosidic bond. The chains are exclusively linear.
Although cellulose is made up of glucose units, but man and carnivorous animals cannot use it as carbohydrate source. Our digestive juices lack the enzyme that can hydrolyse β-1, 4-galactosidic linkages. Carnivorous animals and man need cellulose in the diet to provide bulk for faeces, thus preventing constipation.
Many microorganisms and herbivorous animals (cow, horse, and sheep) can digest cellulose. The higher animals can do so only because they contain microorganism in their stomach (ruminant stomach) which can digest the cellulose.