In this article we will discuss about:- 1. Definition of Proteins 2. Classification of Proteins 3. Composition of Protein Molecule 4. Primary Structure 5. Secondary Structure 6. Quaternary Structure.
Definition of Proteins:
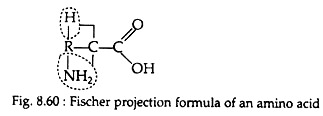
Proteins may be defined as high molar mass compounds consisting largely or entirely of chains of amino acids. The general formula of a naturally occurring amino acid may be represented with the following Fischer projection formula (Fig. 8.60). In this structure an amino group is present on the carbon atom adjacent to carboxyl group.
The amino acids having this general formula are known as alpha (α) amino acids. In its structure four bonds of a carbon atom (Cα) are occupied by NH2, COOH, H and R molecules. R may be any compound which determine the properties of an amino acid and is referred to as the amino acid side chain:
ADVERTISEMENTS:
Classification of Proteins:
Like carbohydrates and lipids, proteins could not be classified only on the basis of structural similarities. Because protein molecules posses great structural complexities.
In earlier days, a convention of classifying proteins was under two headings:
(1) Fibrous—elongated proteins, e.g., silk fibroin, keratin etc.
ADVERTISEMENTS:
(2) Globular—spherical, compact proteins, e.g., egg albumin, caesin and most enzymes. Fibrous proteins tend to be insoluble in water and other solvents, whereas globular proteins are soluble in water and in solutions of salt and water.
Nowadays, there are two alternative methods of protein classification:
(1) According to the composition of the protein and
(2) According to the function of the protein.
ADVERTISEMENTS:
These two classifications are given here:
1. Classification according to the composition:
In this process, proteins are classified into two groups:
(a) Simple proteins, and
(B) Conjugated proteins.
A. Simple proteins:
A simple protein is composed of only α-amino acids. Thus, produces exclusively α-amino acids upon hydrolysis. These proteins are further subdivided according to their solubility in various solvents. Different simple proteins are described here.
(i) Albumins:
These are soluble in water and in dilute salt solutions. Albumins constitute the most important and the most common group of simple proteins. These are present in egg white (egg albumin) and in blood (serum albumin).
ADVERTISEMENTS:
(ii) Globulins:
These are insoluble in water but are soluble in dilute salt solutions. They are widely distributed group of simple proteins. They are present as antibodies in blood serum and as blood fibrinogen.
(iii) Histones:
They are soluble in water and insoluble in dilute ammonium hydroxide. Histones contain a high proportion of basic amino acids (lysine and/or arginine). These are found in association with nucleic acids in the nucleoprotein of the cell.
(iv) Scleroproteins (albuminoids):
These are characterised by their insolubility in water and other solvents. Scleroproteins have structural and protective functions in the body. The examples of scleroproteins are keratin (present in hair, skin and nails), collagen (present in bone, tendon and cartilage) and elastin (elastic fibers of connective tissues).
B. Conjugated proteins:
These are formed of a-amino acids and a non-protein material. The non-protein material of the conjugated protein is called prosthetic group. Different types of conjugated proteins are subdivided on the basis of their prosthetic group.
Different conjugated proteins are as follows:
(i) Phosphoproteins:
These are composed of α-amino acids and phosphoric acids. So, their prosthetic group is phosphoric acid. Caesin, present in milk, is an important member of this group.
(ii) Glycoproteins:
They contain a carbohydrate or a carbohydrate derivative as prosthetic group. Mucin, a constituent of saliva, is a glycoprotein.
(iii) Chromo-proteins:
Their prosphetic group is a pigment compound. Example: Haemoglobin. Its possesses the iron-containing pigment haemeco-ordinated to the simple protein globin.
(iv) Necleoproteins:
Here prosthetic groups are complex polymers of high molar masses and are called nucleic acids (DNA and RNA). Nucleo-proteins are present in all the cells of plants and animals.
(v) Lipoproteins:
They consist of cholesterol esters and phospholipids attached to the protein molecules. They are frequently classified as compound lipids. Most of the lipid in mammalian blood is transported in the form of lipoprotein complexes. The electron transport system in the mitochondria contains large amount of lipoproteins. These are also found in egg yolk, myelin sheath of nerves and different cell organelles.
2. Classification on the basis of functions:
Proteins are classified into six groups on the basis of their functions:
A. Structural proteins:
These proteins participate in the formation of different body parts. More than half of the total protein of the mammalian body is collagen found in skin, cartilage and bone.
B. Contractile proteins:
These special types of proteins are responsible for the contraction and relaxation of muscle cells, e.g., actin and myosin. These proteins are also present in the unicellular animals.
C. Enzymes:
They represent the largest class of proteins. Nearly 2,000 different kinds of enzymes are known. The enzymes are called biological catalyst and are vital for any activity in the living organism.
D. Hormones:
Many of the hormones are protein in nature, e.g., insulin. Some other hormones are steroid.
E. Antibodies:
Higher organisms (birds, mammals, etc.) produce antibodies to destroy any foreign material (antigen) released into the body by an infectious agent. Gamma globulins are example of antibodies in mammals.
F. Blood proteins:
The albumins, globulins and fibrinogen are the three major protein constituents of blood.
Composition of Protein Molecule: Condensation of Amino Acids:
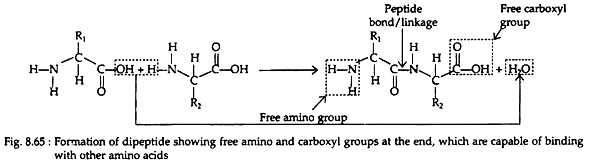
In favourable conditions amino acids polymerize. The a-amino group of one molecule joins to the carboxyl group of another in a condensation reaction. This results in the formation of peptide linkage (Fig. 8.65). The peptides containing two, three and four amino acids are called dipeptides, tripeptides and tetra-peptides, respectively. The amino and carboxyl groups at opposite end of the depeptide can form peptide linkages with other amino acid molecules.
The term polypeptide is generally applied to the relatively small molecules of proteins containing from five to thirty-five amino acids. The term protein is arbitrarily used for longer polypeptides (i.e., having molecular mass above 5,000). However, this is not the fact that protein is composed of only one long polypeptide chain.
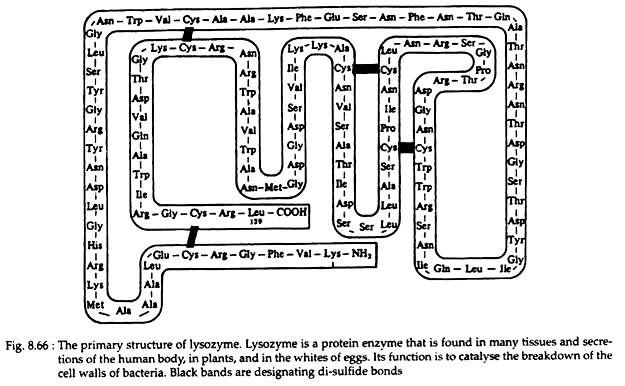
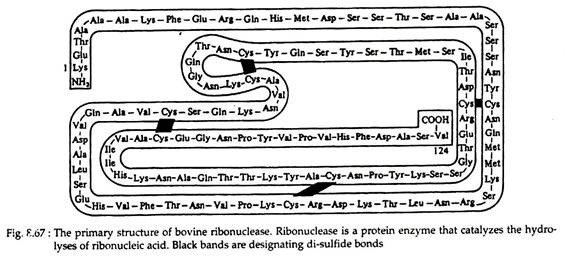
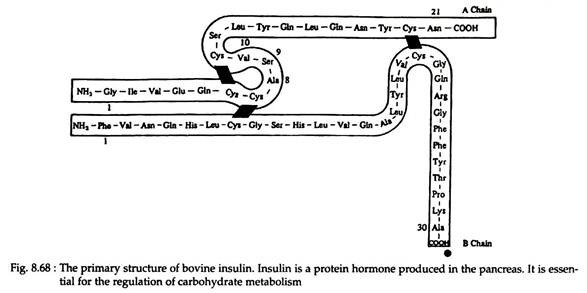
There are also many instances that a protein is composed of two or more polypeptide chains. Example—enzymes lysozyme (Fig. 8.66) and ribonuclease (Fig. 8.67) contain 129 and 124 amino acids, respectively, in only one chain. Hormone insulin (Fig. 8.68) contains two polypeptide chains (one chain having 21 amino acids and the other 30) joined by disulfide linkages. The haemoglobin molecule contains four polypeptide chains.
Primary Structure of Protein:
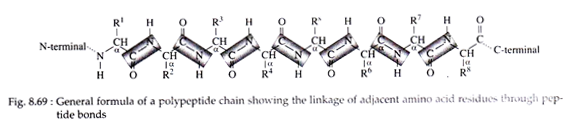
The primary structure of the protein is the number and sequence of amino acids in a polypeptide chain. The only form of bonding in a protein’s primary structure is the peptide linkage. By convention, we represent the structure of peptides beginning with the amino acid whose amino group is free and this end is called as N-terminal end (Fig. 8.69).
The other end, therefore, contains a free carboxyl group and is referred to as C-terminal end. Each amino acid in the peptide, with the exception of the C-terminal amino acid, is named as an acyl group in which the suffixine is replaced by-yl (e.g., alanine → alanyl, glycine → glycyl etc.). Oxytocin and vasopressin are peptides produced by pituitary gland. These are with primary structural configuration.
At present protein research yields a large number of sequences. This number is rapidly growing day by day. So instead of recording them on paper these are deposited in electronic media. There are several protein sequence databases that can be accessed via internet.
Important continuously updated protein databases include EMBL (European Molecular Biology Laboratory Data Library), Gen Bank (Genetic sequence Databank), and PIR (Protein Identification Resource Sequence Database).
Secondary Structure of Protein:
The primary structure describes only the sequence of amino acids in the protein chain. But from the primary structure one cannot understand about the shape (conformation) of the protein molecule. Now it is established that each protein occurs in nature in a single, particular, three dimensional conformations. The fixed configuration of the polypeptide backbone is referred to as the secondary structure of the protein.
Configuration and Conformation of Proteins:
Two considerations are involved in the secondary structure of proteins. The first one is configuration, which refers to the geometric relationship between a given set of atoms. Inter-conversion of configurational alternatives (e.g., conversion of D- to L-alanine) can be achieved only by breaking and reforming covalent bonds.
The second is conformation, which refers to the three dimensioned architecture of the protein, the spatial relationships of all the atoms to all the others. The inter-conversion of conformers involves not the rupture of covalent bonds but the rupture and reformation of non-covalent forces (hydrogen bonds) that stabilise a given conformation.
The particular conformation of a protein is of immense importance in biological activities. There may be different conformational species for a particular protein, but all cannot equally participate in biological actions.
Quaternary Structure of Protein:
Primary, secondary and tertiary structures, described so far, deals with a single helix or one polypeptide chain. The quaternary structure of a protein defines the structure resulting from interactions between separate polypetide units of a protein containing more than one submit.
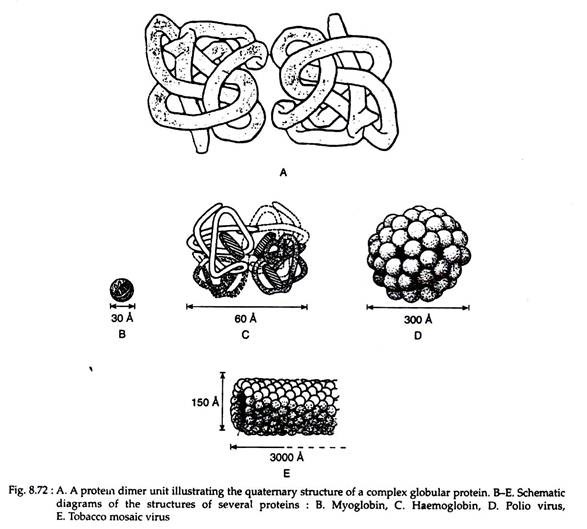
Example, the enzyme phosphorylase A contains two identical submits that alone are catalytically inactive but when joined as a dimer it becomes an active enzyme (Fig. 8.72A).
When this type of proteins is formed of identical subunits, the structure is called a homogenous quaternary structure; if the subunits are dissimilar, a heterogenous quaternary structure is obtained. Another term employed to describe the sub-units of such a protein is promoter, and a protein made up of more than one promoter would be an oligomeric protein. In specific terms haemoglobin is an oligomeric protein having a heterogenous quarternary structure, consisting of two identical α-chain promoters and two identical β-chain promoters (i.e. α2β2) (Fig. 8.72B-D).