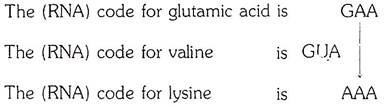Differentiation is a term that is somewhat ambiguous, as it is used at least in two senses – a broader, more general sense and a narrower sense. In the broad sense, differentiation is the process in which the cells or other parts of an organism become different from one another and also different from their previous condition. For example, while the neural plate as a whole is being induced by the roof of the archenteron, its cells become different from both the presumptive epidermis and the parts of blastoderm which gave rise to the neural plate.
In this broad sense, almost the whole of development may be said to be essentially a process of differentiation, and if an understanding is gained of how cells of the embryo become different from one another and from their original condition, then such an understanding would be equivalent to understanding development, or at least a very essential part of it.
At the same time, the term differentiation is also used in a narrower sense, in the sense of histological differentiation. Histological differentiation is the process as a result of which the parts of the organism acquire the ability to perform their special functions. In the case of multicellular animals, the parts in question are the cells and groups of cells.
The special functions of cells in this definition are distinguished from the basic functions of life which are common to all living cells. Every cell is capable of performing the processes of metabolism (respiration, synthesis, and so on), possesses to a certain degree the ability for ameboid movement, shows irritability, and is able to react to external stimuli. These functions are found in both undifferentiated and differentiated cells.
ADVERTISEMENTS:
Differentiated cells, however, are able to perform special functions or to perform them in such a way that other cells cannot. Thus, the nerve cells are capable of conducting nervous impulses to great distances and at a high speed. The liver cells secrete bile (besides their other functions). The melanophores produce granules of pigment in their cytoplasm. These are the special functions of nerve cells, hepatic cells, and melanophores.
The neural plate of the early embryo, although differentiated in a general way, having become different from other parts of the embryo, is not differentiated histologically, since its cells are not yet capable of functioning as nerve cells.
The ability to perform special functions is dependent on the existence of specific mechanisms in the differentiated cells. These mechanisms are sometimes visible in the form of organelles of the cell, such as the myofibrils of muscle cells, the cilia of epithelial cells of the trachea, and the long processes of nerve cells.
These tangible morphological properties of the cells are also called differentiations, a practice that is legitimate as they are actually the visible expression of the process of differentiation.
ADVERTISEMENTS:
In other tissues, the differentiation becomes visible not so much as a change in the structure of the cell itself but as the result of the production by the cells of intercellular structures, such as fibers in 574 the connective tissues, matrix of cartilage and bone, and cuticle on the outer surface of the skin in invertebrate animals.
These extracellular parts are called differentiations in the same sense as the organelles of the cells. The special function of a cell may be the secretion of some substance that does not remain in the tissue as a permanent part but is removed or dissolved in the surrounding medium. In this case, granules of secretion may sometimes be seen in histological preparations as a morphological expression of the function of the cell.
The functional mechanisms of histologically differentiated cells are cytoplasmic. The building up of these mechanisms therefore causes a shift in the relative volume of the nucleus and the cytoplasm. The nuclei of the cells are their conservative part; they do not change essentially with the onset of development.
It is believed that even in the case of fully differentiated cells, the nuclei in all the various tissues of the animal’s body have the same chromosomes and genes. In differentiated cells, the mass of cytoplasm increases while the nuclei do not increase or do not increase in the same proportion.
ADVERTISEMENTS:
The ratio—mass of cytoplasm to mass of nucleus—increases with differentiation. This ratio gives a rough quantitative estimate of the degree of differentiation. The estimate may be made even more exact if chemical substances and not morphological parts are taken into consideration.
The basic substances of the nucleus are the chromosomes, with deoxyribonucleic acid as their essential component. The cytoplasmic structures are composed of proteins. Furthermore the enzymes, which are the essential part of the functional mechanism of a cell, are of a protein nature.
It is thus possible to substitute the amount of deoxyribonucleic acid for the basic structure of the cell and the amount of protein for its changing functional mechanisms. Direct measurements show that the relation, protein/deoxyribonucleic acid, changes with differentiation in agreement with expectation.
Whatever the type of differentiation of the cell, it is doubtless based on the chemical constitution of the cell. In every case in which the function of the cells consists in the elaboration of some substances, whether in the form of structural elements or in the form of secretions, there must be a specific enzyme or specific combination of enzymes responsible for the reaction.
As the substances produced by cells are very diverse, a correspondingly varied assortment of enzymes may be postulated as being present in different tissue cells. It may therefore be said that “differentiation is the production of unique enzymatic patterns”.
All enzymes are known to be proteins, so that the last sentence may be paraphrased to read – “differentiation is the production of unique protein patterns.” This wording is a more general one, as it includes not only proteins having enzymatic properties but also the proteins which are not enzymes. An important group of the latter kind is the structural proteins, which are particularly prominent in some differentiations of cells and tissues.
Some of the structural proteins are permanently intracellular, such as the keratin produced in the epidermis cells of terrestrial vertebrates. Other structural proteins, although produced by the cells, are eventually extruded from the cells and accumulate in the intercellular spaces. Collagen is the best known of such proteins.
Other organic substances, such as lipids and carbohydrates, alone or in conjunction with proteins, play a very important role in the life of cells, but between these substances and proteins there is a significant difference. The synthesis of carbohydrates, lipids, and also smaller molecules, such as organic acids (including amino acids), occurs under the control of enzymes and enzyme systems, which are of a protein nature.
Given a certain pattern of proteins in a cell, the other components are then determined by the enzymatic activities of the proteins (provided, of course that the necessary precursors are present and the environmental conditions are of a specified nature).
ADVERTISEMENTS:
The proteins of the cell, however, are not produced by the action of other proteins, but are synthesized according to a code contained in the DNA of the chromosomes, which is carried to the sites of protein synthesis by messenger RNA. The molecules of messenger RNA are molded on the DNA molecules much in the same way that DNA replicates itself.
The two strands of DNA become separated from each other, and then instead of picking up deoxyribonucleic acid mononucleotides, one of the DNA strands serves as a template for the fixation of ribonucleic acid mononucleotides. The assembly of these mononucleotides into a continuous chain molecule is assisted by an enzyme, RNA polymerase.
In the synthesis of the ribonucleic acid molecule the complementarity of the bases is made use of, just as in the replication of DNA, except that uracil, not thymine, pairs with adenine. In this way the molecule of RNA, being complementary to a DNA molecule, comes to contain the complete genetic information of the latter.
The RNA molecule then splits off from the DNA molecule and, travels from the nucleus into the cytoplasm (probably making use of the pores in the nuclear membrane!), and in the cytoplasm it becomes associated with a ribosome or rather with a group of ribosomes. The messenger RNA molecule is then ready to direct the arrangement of amino acids in the formation of a particular protein molecule.
The base triplets of the messenger RNA correspond in arrangement to the base triplets of the chromosomal DNA. The corresponding amino acids are placed opposite the base triplets of the messenger RNA and are joined together to produce a protein of a specific kind. From what has been said, it is evident that the “genetic code” in the DNA determines the sequence in which the amino acids are joined together to form a polypeptide chain.
The functional properties of the proteins depend largely on the way in which the polypeptide chain is folded to form a three- dimensional structure, the parts of which are held together by cross-linkages. The folding of the polypeptide chain to produce the final structure of the protein molecule is thought to occur spontaneously, given the particular sequence of the amino acid residues in the chain.
Genes Determining Protein Structure – The Hemoglobins:
The relationship between gene structure and protein structure has so far been best studied in the case of hemoglobins contained in the red corpuscles of human blood.
By applying methods of chemical analysis (stepwise fractionation and analysis of fractions) on the one hand, and methods of x-ray diffraction (analysis of x-ray scattering by hemoglobin in crystalline form) on the other, it has been possible to get a complete picture of the organization of the molecules of human hemoglobin.
The hemoglobin—or, to be more exact, hemoglobin A—which makes up about 95 per cent of the hemoglobin of normal human blood, has a molecular weight of about 65,000. The molecule is complex and consists of four subunits – there are two identical a subunits and two identical β subunits, lying in a tetrahedral arrangement.
The whole molecule is roughly spherical. Each of the subunits is a polypeptide chain, folded in a complicated three-dimensional figure, to which is attached a disc-shaped “heme” group carrying in its center an atom of iron, which serves for the binding of oxygen in oxy-hemoglobin.
The polypeptide chain consists of a number of amino acids linked by typical peptide bonds. The exact composition, that is, the sequence of amino acids, is known for both kinds of subunits. The α subunit, apart from the heme group, consists of 141 amino acids; the β subunit consists of 146 amino acids.
In addition to the normal hemoglobin A, other kinds of hemoglobins also occur in man. Of particular interest in connection with gene action are the hemoglobins called hemoglobin S and hemoglobin C. Hemoglobin S has been found to occur in persons suffering from an inheritable condition known as sickle-cell anemia, a disease which in some cases may be fatal.
The red blood corpuscles of persons suffering from sickle-cell anemia have a shrunken, irregular shape often resembling a sickle—hence the name. The condition is inherited in a typical Mendelian fashion and is dependent on a recessive gene, which is allelic to one of the genes involved in determining the normal condition of the red blood corpuscles.
It was found by Pauling and his collaborators (1949) that the hemoglobin S contained in the blood of persons suffering from sickle-cell anemia differs from normal hemoglobin A. The difference is, however, only in one kind of subunit in the hemoglobin molecule, namely, in the β subunit which is changed from βA to βs, while the α subunit is identical with the subunit of normal individuals.
Furthermore, it was found that persons heterozygous for the gene of sickle-cell anemia do not show the symptoms of this abnormality either in their physical state or in the shape of their red blood corpuscles, but their blood contains both hemoglobins A and S in roughly equal proportions.
The presence of subunits βA and βs in the two kinds of hemoglobin indicates that in the heterozygous state the sickle-cell gene and its normal allelomorph produce polypeptide units independently of each other, and these then combine with the a polypeptide units which are not affected by the sickle-cell gene.
This situation indicates a direct relationship between the gene and the protein (or polypeptide) chain which is synthesized under the gene’s control, presumably through the separate emission of a specific messenger RNA by each gene, which then serves as a template for the assembly of the protein chain.
Another abnormal hemoglobin; hemoglobin C, does not cause any deficiencies in persons carrying the gene and can only be discovered by a physicochemical investigation of the blood. The action of the gene causes an alteration of the β component of hemoglobin, while the α component remains unchanged, as in sickle-cell disease. The inheritance of the condition closely resembles the inheritance of sickle-cell anemia; the gene is allelic to the gene responsible for the formation of component β of normal hemoglobin.
In persons homozygous for gene Hb-C, most of the hemoglobin molecules have the composition α2A + β2c; in heterozygous persons, roughly half the molecules have this abnormal composition, and the other half have the normal structure α2A + β2A. Again, the presence of both kinds of β chains in heterozygous persons shows that each gene acts independently in producing the specific kind of polypeptide molecule in the cell containing such a gene.
A further understanding of the close relation between the gene structure and the structure of the corresponding protein emerges from a study of the amino acid composition of the abnormal hemoglobins S and C. By comparing the amino acid sequence in the β chains of normal hemoglobin A and of the abnormal hemoglobins S and C, it was found that the latter two differ from normal hemoglobin only in one amino acid.
In sickle-cell hemoglobin, the glutamic acid residue which is the sixth from the beginning of the β chain is replaced by a valine residue. In hemoglobin C the same glutamic acid residue is replaced by lysine.
The section of the β chain in which the replacement occurs can be shown as follows:
Normal hemoglobin A, β chain- Val-His-Leu-Thr-Pro-Glu-Glu-Lys
Hemoglobin S, β chain- Val-His-Leu-Thr-Pro-Val- Glu-Lys
Hemoglobin C, β chain- Val-His-Leu-Thr-Pro-Lys-Glu-Lys
According to the “genetic code”:
It is evident that a change in one base only could account for the transformation of the normal hemoglobin into one or another of the alternative forms. In the case of sickle-cell hemoglobins, a uracil nucleotide could have been substituted for an adenine nucleotide; in the case of hemoglobin C, an adenine nucleotide could have been substituted for a guanine nucleotide.
The abnormal hemoglobins S and C most probably owe their origin to mutations. It thus becomes likely that “point” mutations (mutations of a single gene) may, at least in some cases, result from a change in only one base pair of DNA, which changes a code triplet in such a way that it determines a different amino acid from the one coded by the previously existing sequence of base pairs in the chromosomal DNA.
As a result of a change in one code triplet, the resulting protein would have one amino acid residue in the chain replaced by a different one. Sometimes such a replacement might not seriously affect the functional properties of the protein concerned, but occasionally the change may be very considerable.
We have seen that the substitution of only one amino acid residue in the β chain of human hemoglobin not only leads to a distinct morphological expression, in the shape of the red blood corpuscles, but also changes the metabolism of the whole organism. The change in one base pair in the chromosomes may become a matter of life or death for the carrier of the abnormal gene.