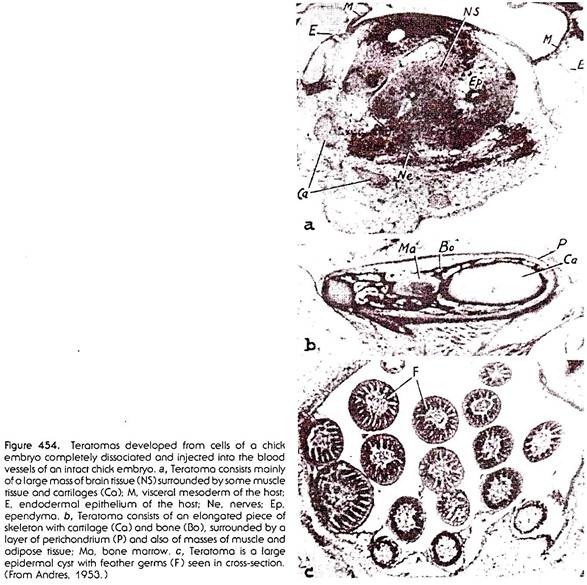
In experiments of disaggregation and re-aggregation of embryonic cells, there sometimes arise complexes in which differently differentiated tissues are present in chaotic arrangement, having no relation, or only a slight relation, to the arrangement of the same tissues in a normal individual. We have referred to such complexes as teratomas (Fig. 454). The term “teratoma,” however, was originally coined to denote certain growths that occur spontaneously in man and animals.
Normal ontogenetic development rests on the co-ordinated sequence of a large number of processes. Differentiation of various tissues occurs in a way that is strictly determined as to time and place, and parts of the embryo of the developing individual grow at rates that are also strictly determined in time and space, and the growth is retarded and stopped in such a way that the “normal” end condition of development becomes established.
The interconnection between the differentiation and growth of parts of the organism is not fixed inseparably, however, and under certain circumstances the interconnection may be broken, and partial developmental processes proceed without relation to others that accompany them in normal ontogenesis.
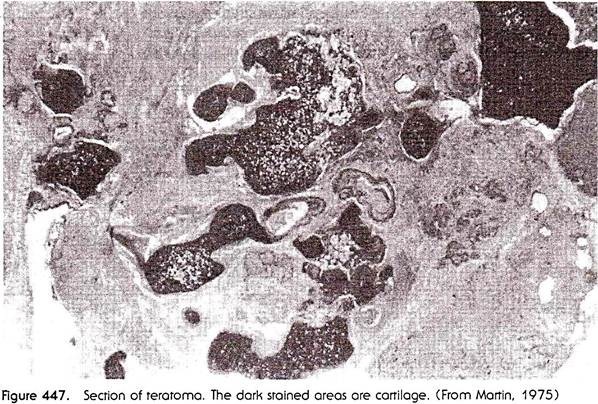
In humans, teratomas are quite frequently encountered as non-malignant growths on the ovary. The growth, which sometimes attains cocoanut size, is composed of a variety of tissues, including practically all that occur in a normal body, arranged without any order in space. The tissues include skin, with skin glands and hairs, tissues of the alimentary canal, internal glands, lung and kidney tissues, bone and cartilage (which, however, are never shaped as recognizable skeletal elements), muscle, and nervous tissue (Fig. 447).
Well-developed teeth are often present, and also, sometimes, embryonic tissues such as chorion. Teratomas also are found sometimes as outgrowths of the testes, but in these cases the differentiated tissues similar to those in ovarian growths are almost always accompanied by masses of undifferentiated cells, which show unlimited—that is, malignant—growth. The teratoma thus becomes a teratocarcinoma.
Teratomas also occur spontaneously in animals other than humans. In recent years much work has been done on teratomas and teratocarcinomas in mice in which some genetic strains have been producing these abnormal growths in a particularly high proportion of individuals. In one genetic strain about 50 per cent of females develop ovarian teratomas, and in another genetic strain 12 per cent of males produce testicular teratomas. Most of these teratomas are non-malignant.
ADVERTISEMENTS:
There are several ways of inducing teratomas experimentally. One way of doing this is by chemical treatment. It has been found that injecting a 5 per cent solution of zinc chloride into the testes of cocks causes the development from the testicular tissues of a typical teratocarcinoma containing not only a variety of differentiated tissues, but also indefinitely proliferating malignant cells.
A second method of producing teratomas and teratocarcinomas artificially is to transplant in mice, the genital ridges of 12-day fetuses into the testes of adult mice. Up to 75 per cent of such grafts produce teratomas and teratocarcinomas similar to those arising spontaneously. The third method, also applied in mice, is to transplant a young mouse embryo, at any stage between two blastomeres and the seventh day of development, into an extrauterine site such as the kidney or testis of an adult mouse. Up to the seventh day after fertilization the embryo continues to develop more or less normally; however, after that the development becomes disorganized, and the graft continues to grow as a teratocarcinoma.
By examining the fetuses of mice producing a high proportion of teratomas it was established that these can be distinguished already before birth, and that originally they consist of groups of undifferentiated cells inside the gonad rudiments, the rudiments of the testes in particular. In the course of later development these early rudiments give rise to the various kinds of differentiated tissues composing the teratoma.
If all the undifferentiated cells are used up in producing differentiated tissues, the rudiment becomes a benign growth or teratoma. In teratocarcinomas, however, a residue of undifferentiated cells remains, and these continue to proliferate. Teratocarcinomas of this kind are transplantable- that is, if part of such a tumor is grafted to another mouse, the graft develops into a new tumor. Teratocarcinomas may be carried as tissue cultures, and in this form they are similar to cultures of cancer cells.
ADVERTISEMENTS:
A difference between teratocarcinomas and cancerous cells, at least in the initial stages, is that the undifferentiated cells of the teratocarcinomas are able to produce differentiated tissues when transplanted into a new host (another mouse). This ability may be lost, however, after prolonged cultivation in vitro, after which the cells become “nullipotent,” that is, incapable of undergoing any kind of differentiation.
On the other hand those parts of teratocarcinomas which do differentiate lose their malignancy. In this respect teratomas and teratocarcinomas conform to the general principle of differentiation being an antagonist of growth – cells that undergo histological differentiation cease to proliferate, or proliferate at a reduced rate.
The origin of teratomas and teratocarcinomas is clearly connected with gonads — male or female. It is at present accepted that the cells giving rise to these types of growth are actually generative cells. In the testes these are presumably spermatogonia; in the ovaries they may be oogonia, or possibly ova undergoing a parthenogenetic development. The evidence in favor of this view, apart from the origin of the growth in the gonad, is of two kinds.
Firstly, the very early traces of abnormal growth in the testes of mouse fetuses are found actually within the seminiferous tubules, where all the cells (with the exception of the Sertoli cells, which can be left out of consideration) are the primordial germ cells and their descendants. Secondly, there is a mutant strain of mice in which the homozygote individuals are completely devoid of primordial germ cells.
These individuals are at the same time resistant to experimental induction of teratomatous growth. These experiments suggest that the rule is – no germ cells—no teratomas. Germ cells, of course, possess the full genotype, and for this reason it is understandable that in the growths produced by these cells all possible differentiations can be present.
The various potentialities encoded in the genome of cells showing teratomatous growth are, however, realized in a chaotic way, indicating that the genes responsible for the differentiations are activated at random. If it were possible to learn how the activation is achieved in a teratoma, a large step would be made toward understanding gene action in normal development.
It may be presumed that the chaotic and random activation of genes in a teratoma is due to the fact that the starting point of its growth is not a fertilized egg cell, with all the mechanisms contained in such a cell for the orderly course of cleavage, gastrulation, and subsequent organogenesis. This is true, even if the starting point of a teratomatous growth is a transplanted embryo, as the latter becomes disorganized, and the mechanism that had been laid down in the ovum is thus wiped out.
Another difference, which may account for the chaotic differentiation of parts of a teratoma, is that the embryo develops as a self-contained unit, whiles a teratoma, whether a spontaneous or an experimentally induced one, develops in contact and in association with adjoining tissues, and thus is exposed to stimuli from these adjacent tissues.
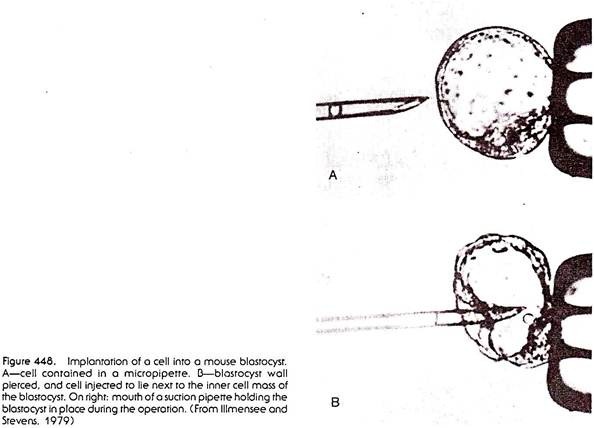
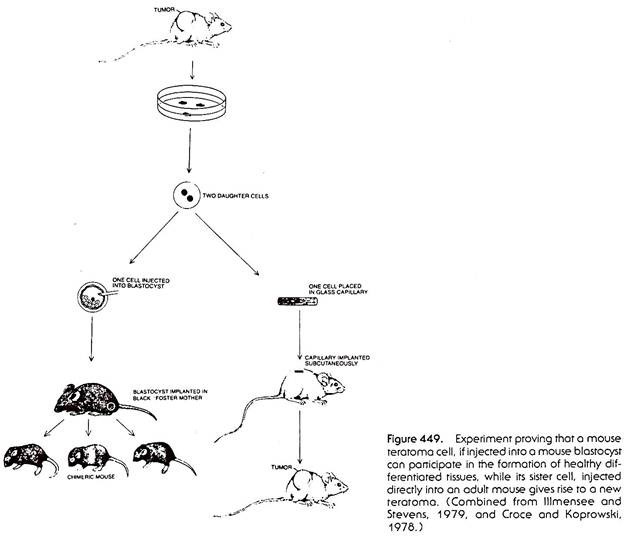
The state of the genome in malignantly growing teratocarcinoma cells was tested in some very interesting and elegant experiments. After dissociation of a teratocarcinoma into single cells by treatment of the tumor with protein-digesting enzymes, single teratocarcinoma cells were injected into mouse blastocysts (Fig. 448). The blastocysts were then introduced into the uterus of pseudo-pregnant foster-mother mice.
To trace the fate of the implanted teratocarcinoma cell and its offspring, the blastocysts were taken from mice of a different color strain than the ones giving rise to the teratocarcinoma. When the blastocyst gave rise to a newborn mouse, and the latter became covered with hair, it was found that parts of the skin grew hair of a color pertaining to the strain from which the teratocarcinoma cell was taken. It was thus evident that the cell implanted into the blastocyst became integrated into the “inner cell mass” of the host blastocyst and, instead of producing a malignant growth, harmoniously participated in the development of the tissues of the host mouse (Fig. 449).
In a control experiment two teratomatous sister cells were separated, and while one sister cell was implanted into a blastocyst and gave rise to normal tissues of the host mouse, the other sister cell was placed in a glass capillary and implanted into an adult mouse, where it gave rise to a tumor. The two sister cells must have had identical genomes, yet the one cell differentiated into normal tissues, and the other remained malignant (Fig. 449).
The final proof that teratocarcinoma cells retain the normal genome intact was given by a further modification of the above experiment. In one case the descendants of teratocarcinoma cells implanted into a blastocyst were incorporated among other tissues also in the ovary of the host mouse and gave rise to eggs.
When fertilized, these eggs developed into normal individuals showing the genetic characteristics of the strain from which the teratocarcinoma was derived. Thus, paradoxically, cells participating in malignant growth retain the potentiality for producing a complete normal animal. The factor which caused the return to normality was obviously the environment and organization of the developing blastocyst, into which the malignant cell became incorporated.
From these experiments it may be concluded that the genome of malignantly growing cells of a teratocarcinoma is not abnormal or substantially impaired, but that the abnormal behavior of these cells rests on a disturbance of the factors regulating the functioning of the genome, that is, on a disturbance of its controlling mechanisms.
Cancer:
A further step in the deviation from normality is found in the malignant growths known as cancer. The origin of cancerous growths is radically different from that of teratomas and teratocarcinomas, inasmuch as they bear no relation to gonads and reproductive cells. Cells of any tissue of the body may become malignant; this means that these cells start proliferating, and penetrating (infiltrating) into surrounding healthy tissues.
The penetration may be effected by individual cells moving between other cells, or, in a more dangerous case, cancerous cells find their way into lymphatic vessels or blood capillaries, and with the lymph and blood become distributed throughout the body of the victim. The cancerous cells may then settle down in sites far removed from the original focus of malignant growth, forming secondary growths, or metastases.
The cells of the malignant growths lose much of the differentiation typical of the tissues from which they are derived, but some biochemical characteristics of the original tissue may be retained. Thus cells of the mammary cancers and cells of prostate gland cancers retain a different reactivity to male and female hormones. Malignant-growths originating from epithelial tissues are designated as carcinomas; those originating from connective tissues and their derivatives are called sarcomas.
Not all abnormal growths are necessarily malignant. We have seen that teratomas may be non-malignant. Some of the growths originating in somatic tissues may also be non-malignant, in the sense that they do not infiltrate neighboring tissues and do not form metastases, but remain restricted to their original location.
There are three ways by which normal cells can become transformed into malignantly growing cells:
(1) Malignant growth may start “spontaneously.” This in fact means that the reason for the origin of cancer is not known.
(2) Malignant growth may be caused by chemical or physical factors. There is a large class of chemical substances which are “carcinogenic.” A large number of these substances are found among the steroids—substances containing several benzene rings linked together.
Some of the chemicals belonging to the steroid group act as sex hormones in vertebrates; others are contained in tars. The presumed danger of cigarette smoking is due to the presence of carcinogenic tars in tobacco. All forms of ionizing radiation—ultraviolet radiation, x-rays, gamma rays emitted by radioactive substances—is, in sufficient doses, capable of causing malignant growths.
(3) Malignant growth may be caused by infection of cells with certain viruses. Perhaps the best known case is that of the “Bittner” virus, which causes mammary carcinoma in mice. The virus is transmitted from an infected mother to its offspring in the milk which the young mice suckle. These offspring become infected with the virus, and in due course mammary tumors develop in a large proportion of these individuals. If newborn offspring of an infected female are separated from their mother, and suckled by a healthy foster mother, they remain free of the infection and do not develop mammary cancers.
Another virus which is carcinogenic is the “simian virus,” designated SV40. This virus has been used to cause malignant transformation of human cells grown in tissue culture. There are a number of other viruses that are carcinogenic.
While retaining some of the specificity of the original tissue, cancerous cells of all types acquire certain peculiar characteristics of their own, which incidentally are also shared by the malignant cells of the teratocarcinomas. One of the characteristics of cancer cells is an absence of so-called “contact inhibition.” When non-malignant cells are cultivated in vitro, and these cells move about and meet one another in the culture medium, they become immobilized at least temporarily and also stop proliferating.
When cancer cells meet under the same conditions, they are not in the least inhibited in their movement, or in their ability to proliferate. This perhaps in part accounts for the facility with which cancerous cells disperse in the organism and infiltrate adjoining healthy tissues. A second characteristic of the cancer cells is that they do not develop “gap junctions” between one another.
Electric currents applied to one of these cells does not spread into adjacent cells, no matter how close they lie together, while propagation of electric currents, and thus ion flow, is the rule with “normal” cells when they are in contact. This may have something to do with the escape of the cancer cells from the controlling influence of the body as a whole – the gradients which control the organization of the early embryo are maintained by means of substances passing from cell to cell, presumably by means of gap junctions.
Both the change in the reaction of cancerous cells to contact with other cells and the failure of these cells to “communicate” chemically with other cells, suggest that the properties of the cell surface are changed when a cell becomes cancerous. In fact, new kinds of proteins appear in the composition of the cell membranes of cancerous cells, which were not there in normal cells of the same kind of animal.
The reader will recall that the cell membrane consists of a double layer of lipid molecules with protein and glycoprotein molecules inserted into the layer of lipids. While lipids in the cell membrane are fairly uniform in different cells and in different animals, it is the protein and glycoprotein molecules projecting from the lipid layer on the surface of the cell membrane that are responsible for the specificity of the cell surfaces of different cells.
The presence of new proteins or glycoproteins on the surface of cancerous cells can be proved in two ways. One method depends on experiments using the strictly inbred strains of animals, which after prolonged brother-sister mating’s become completely identical in their genotypes. Such experiments were done on mice. If a tumor is induced in a mouse of such an inbred strain, a bit of the tumor can be used to immunize mice of the same strain against the tumor.
The cancer proteins on cells of the tumor are recognized by the body of the recipient of a tumor graft as something different from its own body; the whole defense system (involving leukocytes) is directed against the foreign intruder, and the tumor is rejected. The use of inbred strains is important, because otherwise it would not be possible to know whether the rejection is specifically directed against the tumor as such, or whether this is a common case of graft rejection which occurs whenever a graft is made from an individual with different genetic and biochemical properties.
In the other method for proving the existence of new proteins on the surface of cancer cells, an altogether different animal (horse, rabbit) is immunized by injecting parts of a mouse tumor, and thus developing an antiserum to the tumor. The complication of this method is that the antiserum would contain antibodies against the normal tissues of the mouse, in addition to antibodies against the “cancer” proteins; these antibodies against mouse tissue in general must be removed before the serum can be used for the detection of specific cancer proteins. In immunological nomenclature the proteins eliciting immune reactions are termed “antigens.” It will be convenient therefore to refer to the new proteins appearing on cancerous cells as tumor antigens.
The appearance on the surface of tumor cells of proteins that were not present in healthy cells indicates, according to all that we know of protein synthesis, that an alteration in the function of the genome has occurred. There are two possible interpretations of the synthesis of new kinds of proteins. One possibility is that a gene mutation had taken place, either spontaneously (as mutations do occur in nature), or through the action of the mutagenic agent (carcinogenic chemicals, x-rays).
A mutated gene would be able to produce a new kind of mRNA and eventually cause the synthesis of a different sort of protein. The other possibility would be that transformation into a cancerous cell is the result of some alteration of the control mechanisms in the cell, so that parts of the genome normally quiescent are activated, or that sequences of DNA which normally are eliminated in the processing of the heterogeneous nuclear RNA are now incorporated into an altered mRNA, and subsequently released to be translated into new proteins.
In favor of the claim that malignant transformations of cells are a direct result of mutations is the fact that all carcinogenic chemicals are also able to induce mutations of other kinds, at least when they are tested on bacteria. On the other hand, the reverse does not hold- many agents causing mutations are not carcinogenic. It would thus appear that only mutations of a certain special kind lead to cancer.
A change in the control of existing genome sequences, while not being a mutation in the sense of changing the sequence of an existing structural gene, must be the result of some alteration of the genome, presumably in the parts serving for the regulation of gene activity.
It is not possible at present to make a definite decision concerning either of these two possibilities, but there are certain experimental results which have a bearing on the problem.
In one relevant experiment, human cells grown in tissue culture were transformed into malignantly growing cells by infecting them with simian virus (SV40). In the process, the DNA of the virus was incorporated into a chromosome of the human genome. Next, the transformed human cells were somatically hybridized with mouse cells. As happens in this kind of hybridization, the hybrid cells tend to lose part of the human chromosomes.
The loss is random, and the remaining chromosomes could be identified because the human chromosome set has been studied very thoroughly, and each individual chromosome can be recognized. A number of clones of hybrid cells were cultivated and tested for the presence of the tumor antigen and for the ability to produce tumors when injected into mice. Some clones of cells showed the presence of the antigen and could produce tumors, but clones of other cells did not produce tumors and lacked the antigen.
It was found that a particular human chromosome, chromosome 7, had been retained in all tumor-producing clones; however, those clones in which chromosome 7 was lost did not show the presence of the antigen and could not cause tumors.
Furthermore, virus particles could be recovered from cells retaining chromosome 7, but not from others. The experiment proves that the virus genome was incorporated into a particular chromosome, chromosome 7, and that its presence was responsible for the production of the tumor antigen, and for the transformation of healthy cells into malignantly growing tumor cells.
While this experiment proves that the presence of an additional (virus) genome sequence can be responsible for the malignant transformation, not only of the original human cells, but also of the hybrid human-mouse cells, it does not show that the genes of the recipient cells had been changed.
The cell surface antigens present in tumor cells are different depending on the origin of the tumor—on the mechanism of transformation of normal into tumor cells. When tumors are produced by chemical or physical methods, each tumor has a different set of cell surface antigens. This strongly suggests that a random mutation has been evoked in the affected cells’ genomes. The result could also be explained, however, as being due to an activation of genes, or other normally non-meaningful sequences, at the wrong time and in the wrong place, but without an actual alteration of the genome.
When the malignant transformation of cells is due to virus infection, on the other hand, the cell surface antigens which appear are always the same for the same virus. The virus antigens appear without being directly involved in the construction of virus particles (virions). Thus, in mouse leukemia, which is presumably caused by a mouse leukemia virus (MuLV), there are at least three different tumor antigens present- the CGSA antigen, the GIX antigen and the TL antigen. The first two are proteins which also are involved in the formation of the virion, or at least are related to these.
The third antigen has not been shown to be directly related to the virion proteins. The remarkable thing about these antigens is that, in strains of mice which show a high percentage of spontaneous leukemia, the antigens may be found on normal cells in different tissues. In strains of mice that rarely produce leukemia spontaneously, the antigens are not present on healthy cells, but they appear on malignant cells if a tumor is provoked by artificial means (by carcinogenic chemicals or x-rays).
This has led to a rather paradoxical assertion that all mice are infected with the MuLV virus, and that this latent virus may from time to time be activated, either due to a specific genome, as in strains of mice high incidence of “spontaneous” leukemia, or by external factors as in experimental production of the disease! Because the cell surface antigens appearing in this type of leukemia are always the same, there is little ground to assume that new genetic factors – new mutations – are responsible for the flare-up of the malignant growth. Rather, it would seem that the malignant transformation is due to a disturbance on the normal gene control.
A case somewhat similar to that of the mouse leukemia virus is suspected in a disease in humans caused by a virus – the “Epstein-Barr” virus. This virus causes malignant growth in the submandibular lymphatic glands. The disease, known as “Burkitt’s lymphoma” is found quite frequently among small children in tropical Africa. The presence of the virus may be discovered because antibodies against the virus are produced by infected persons, and these antibodies circulate in the blood and may be easily detected.
It was discovered that antibodies against the Epstein-Barr virus are present not only in patients that are suffering or have suffered from Burkitt’s lymphoma but in practically all small children in tropical Africa, in spite of their showing no symptoms of the disease.
Furthermore, it was discovered that antigens against the Epstein-Barr virus are present in blood of persons living in areas other than tropical Africa – but in adolescents, rather than in small children. Outside of Africa the virus is occasionally supposed to cause a disease (not a tumor!) called ‘infectious mononucleosis.’ This disease manifests itself by fever and swelling of lymph glands on the neck. It is not a serious illness and passes spontaneously.
As with mouse leukemia, the Epstein-Barr virus seems to be present in most humans but in the overwhelming majority of infected persons it does not cause any discomfort whatsoever (though its presence is revealed by the antibodies against the virus in the blood!); in some it causes the mild disease of infectious mononucleosis; and, only under special circumstances, in the environment of tropical Africa, it produces malignant growth. The presence of the virus is not sufficient to produce disease – some additional factors must be added to transform the potentiality embodied in the DNA sequence of the virus genome into the actuality of disease or tumor.
In a certain mouse liver tumor an antigen is produced which circulates in the blood of the cancerous mice. The antigen can be detected because an antibody to it may be produced by immunizing rabbits with the blood from the affected mice. The same antigen was detected in the blood of healthy fetal mice. The antigen was therefore named the alpha-fetoprotein.
The protein disappears from the blood of healthy mice shortly after birth. As the synthesis of the protein must have been the end-result of the activity of a specific gene, it follows that in malignant transformation of liver cells a gene that should have been inactive in normal life is reactivated—again an indication that cancer is the result of change in the regulation of gene activity.
Because in mice the leukemia virus is presumably always present but becomes active only under certain conditions (and the same seems to be also true for the Epstein-Barr virus in humans), the extrapolation could be made that malignant growth is always in the last instance due to virus infection, which flares up only sporadically under internal or external stimulation. It would be very difficult to either prove or disprove such an assumption.
Some further insight into the genesis of tumors would be achieved if the cell surface tumor antigens could be traced back to their origins and their chemical structure could be known; if the messenger RNA’s responsible for their synthesis could be isolated, and if their relation to the chromosomal DNA could be clearly established, not only in virus-induced tumors, but also in tumors resulting from chemical and physical treatment.