The following points highlight the six main types of cloning vectors. The types are: 1. Plasmid Vectors 2. Bacteriophages as Vectors 3. Cosmids as Vectors 4. Phagemids as Vectors 5. Vectors for Cloning Larger DNA Fragments 6. Vectors for Preparing Single-Stranded DNA.
Type # 1. Plasmid Vectors:
The first vector that was developed for gene cloning was plasmids which are versatile and thus widely used. Plasmids are naturally occurring extra chromosomal, double-stranded DNA molecules that replicate autonomously within bacterial cells (Fig. 9.6).
Their replication depends on the same enzymes that replicate the chromosome of the host cells, and they are distributed to daughter cells along with the host chromosome during cell-division. Plasmids range in size from a few thousand base pairs to more than 100 kilo-bases (kb).  Like host-cell chromosomal DNA, plasmid DNA is duplicated before every cell division. During cell division, at least one copy of the plasmid DNA is segregated to each daughter cell, assuring continued propagation of the plasmid through successive generations of the host cell. Most plasmids are circular but linear plasmids have been found in some bacteria like Streptomyces and Borrelia burgdorferi.
Like host-cell chromosomal DNA, plasmid DNA is duplicated before every cell division. During cell division, at least one copy of the plasmid DNA is segregated to each daughter cell, assuring continued propagation of the plasmid through successive generations of the host cell. Most plasmids are circular but linear plasmids have been found in some bacteria like Streptomyces and Borrelia burgdorferi.
Many naturally occurring plasmids contain genes that help the host cells. For example, some bacterial plasmids encode enzymes that inactivate antibiotics. Therefore, a bacterial cell containing such plasmid is resistant to the antibiotic and can replicate in an environment containing the antibiotic, whereas the same type of bacterium lacking the drug-resistant plasmid is killed. Such property of plasmid is exploited in their use as cloning vectors.
ADVERTISEMENTS:
Plasmids may be:
(i) Single copy plasmids that are present as one plasmid DNA per cell,
(ii) Multi-copy plasmids which are maintained as 10-12 genomes per host cell and
(iii) Plasmids under relaxed replication control, which can increase their copy number in the host cell up to 100 copies. These plasmids are widely used as cloning vectors.
ADVERTISEMENTS:
However, to act as cloning vectors, all plasmids should possess the following properties:
(i) Plasmid can be easily isolated from their host cells.
(ii) Plasmid should have single restriction site for one or more restriction enzyme(s)
(iii) Insertion of a linear foreign DNA at one end of these sites should not alter its replication properties and
ADVERTISEMENTS:
(iv) Plasmid can be reintroduced into the host bacterial cells. Cells carrying the plasmid with or without the insert can be identified or selected.
Modifications of plasmids:
Most plasmids used commonly in recombinant DNA technology, replicate in the bacterium E. coli. A single plasmid may not possess all the characteristics required for easy DNA cloning. Therefore, these plasmids have been engineered (genetically modified) to optimize their use as vectors in DNA cloning.
For instance:
(i) Their length is reduced to simplify working with plasmids; many such plasmids are only =3 kb in length, much less than their natural lengths (the circumference of plasmids is usually referred to as their ‘length’ even though they are almost always circular).
(ii) Most plasmid vectors contain little more than the essential nucleotide sequences required for their use in DNA cloning: a replication origin, a drug-resistance gene and a region in which exogenous DNA fragment can be inserted.
(iii) Plasmids are also modified to increase their copy number in host cells by a process of amplification.
ADVERTISEMENTS:
(iv) Plasmid vectors can be constructed with a poly-linker or multiple cloning sites (MCSs).
An MCS is a short DNA sequence, 2.8 kb in case of PUC 19, carrying sites for many different restriction endonucleases. An MCS increases the number of potential cloning strategies available by extending the range of enzymes that can be used to generate a restriction fragment suitable for cloning. By combining them within an MCS, the sites are made contiguous, so that any two sites within it can be cloned simultaneously without excising vector sequences.
Examples of some plasmids:
(a) pBR Vector Plasmids:
In cloning experiments, the pBR group of plasmids is the most widely used cloning vectors. Among these, pBR322 has been completely sequenced through modification of earlier plasmids of E. coli, pBR318 and pBR320 (The pBR was named after the discoverer of these plasmids; Bolivar and Rodriguez).
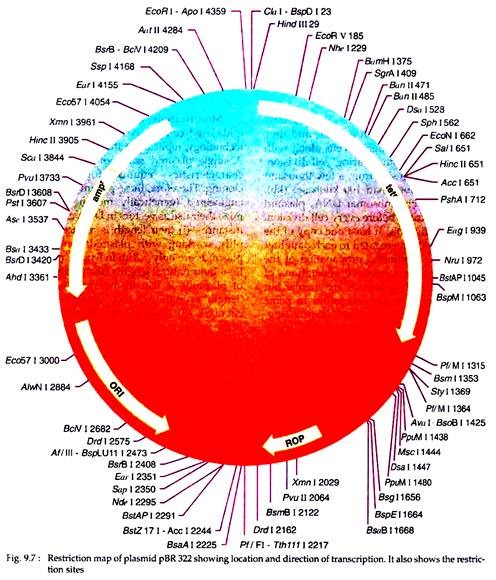
In addition to its use as cloning vehicle, the pBR322 is also widely used as a model system for the study of prokaryotic transcription and translation, as well as investigation of the effects of topological changes on DNA conformation. The pBR322 plasmid is 4362 bp in length and has an origin of replication (ori) that has derived from a plasmid related to naturally occurring plasmid ColEI.
It also possesses genes conferring resistance to antibiotics, e.g., ampicillin (ampr) and tetracyclin (tetr). There are over 10 enzymes with unique cleavage sites on the pBR322 genome (Fig. 9.7). The target sites of these enzymes lie within the tetracyclin resistant gene (tetr) and there are sites for further two (ClaI and Hindlll) within the promoter of that gene. Six unique restriction sites lie within the ampr gene.
Thus, cloning in pBR322 with the aid of any one of these enzymes will result in insertional inactivation of either the ampr or the tetr markers. The recombinant plasmid will then allow the host cells to grow in the medium only in presence of ampicillin or tetracyclin but not in presence of both.
However, cloning in the other unique sites does not permit the easy selection of recombinants, because neither of the antibiotic resistance determinants is inactivated.
In pBR322, the PstI site in the amp’ gene is particularly useful, because the 3′ tetra-nucleotide extensions formed on digestion are ideal structures for terminal transferase. Thus, this site is excellent for cloning by the homopolymer tailing method as described earlier.
Cloning into Hindlll site of pBR322 generally results in loss of tetracycline resistance. However, in some recombinants tetr is retained or even increased. This is due to the presence of Hindlll site within the promoter rather than the coding sequence. Thus whether or not insertional inactivation occurs depends on whether the cloned DNA carries a promoter like sequence able to initiate transcription of the tetr gene.
pBR322 as the source of other improved vectors:
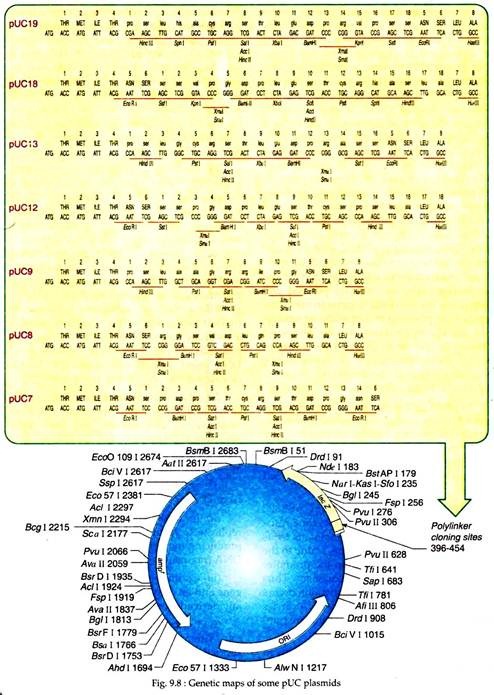
Over the years, many advanced plasmid vectors have been constructed from pBR322 through insertion of additional unique restriction sites and selected markers. For example, pBR325 plasmid encodes a chloramphenicol resistance in addition to ampicillin and tetracycline resistance and has a unique EcoRI site in the cm’ gene. Now a day’s construction of new vectors is done by use of poly-linkers or multiple cloning sites (MCSs) as exemplified by the pUC vectors (Fig. 9.8).
Another vector pBR327 was derived from pBR322 by deletion of nucleotides between 1427 to 2516 to reduce the size of this vector. This eliminated nucleotide sequences were also known to interfere with the expression of the cloned DNA in eukaryotic cells. However, pBR327 plasmids also possess the two antibiotic resistance genes, ampr and tetr.
Therefore, the insert bearing plasmids can be selected by their ability to grow in a medium containing only one of two antibiotics and by their failure to grow in a medium having both the antibiotics. The plasmids, on the other hand, containing no DNA insert, will be able to grow in media containing one or both the antibiotics.
(b) pUC Vector Plasmids:
These genetically modified plasmids were first prepared in University of California and thus, were named pUC (Plasmid University of California (Fig. 9.8).
These plasmids possess several advantages as a vector for cloning like:
(i) Small size but can carry relatively large DNA inserts.
(ii) In a host cell, pUC like pUC18 can replicate to form about 500 copies per cell, producing many clones or copies of inserted DNA fragments.
(iii) Contain one ampicillin resistance gene and an origin of replication that is usually derived from E. coli plasmid pBR322.
(iv) Contain a lac Z gene derived from E. coli. Within the lac region a large number of restriction enzyme sites have been engineered, called a multiple cloning poly-linker site.
(v) pUC plasmids have a selection system that can distinguish between recombinant (plasmid with inserted DNA fragment) and non-recombinant plasmids. For example, in the plasmid pUC18, when DNA fragments are inserted in the lac region, the lac gene is inactivated Such plasmids when transformed into the host E. coli strain (lac–) and grown in presence of IPTG (isopropyl thiogalactoside, which induces the synthesis of β galactosidase enzyme like lactase) and X- gal (substrate for β galactosidase), a white or colourless colonies will form.
When pUC18 having no inserts are transformed into host bacterial cells, through the action of lac Z- gene, bacterial host cells will produce blue colonies. This permits easy identification of pUC18 vectors having cloned DNA segments from those having no cloned DNA fragments. Plasmids belonging to pUC family are available in pairs having reverse order of restriction sites relative to lac Z promoter, like pUC8 and pUC9, pUC12, pUC13; pUC18 and pUC19.
Type # 2. Bacteriophages as Vectors:
Bacteriophages or phages are viruses that infect bacterial cells. Some phages are widely used as cloning vectors. Because of small size, plasmids are best used for single gene insertion and the insert will rarely be larger than about 2 kb.
But for cloning of larger pieces of DNA (e.g., for genomic library preparation), the plasmids are not suitable as larger inserts will increase the plasmid size making them unsuitable for transformation.
The phage has a linear DNA molecule which will produce two fragments following a single break. The fragments can be joined with foreign DNA and the chimeric phages thus produced can be isolated after allowing them to infect bacteria and collecting progeny particles after a lytic cycle.
However, in order to optimize the insertion capacity, phage DNA itself may be modified according to the purpose. Several phages used commonly in DNA cloning are described below in brief.
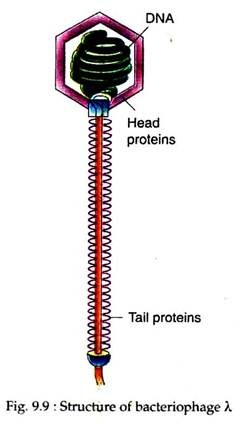
Phage λ as Vector: The most extensively studied bacterial virus used for DNA cloning is bacteriophage λ (Lambda). The λ phage virion has a head region containing the viral DNA genome, and a tail which functions in infecting its host, the E. coli (Fig. 9.9).
The wild-type A virus particle or virion contains a 50 kb liner double-stranded DNA as its genome which is packaged within a protein coat. When a virion attaches itself to the host bacterial cell, the coat protein is discarded and A DNA is injected into the cell.
At the extreme termini of the λ DNA are overhanging 5′ ends which are 12 nucleotides long, and complementary in base sequence. These large 5′ overhangs can base- pair, and are effectively sticky ends, similar to, but more cohesive than the small sticky ends generated by some restriction nucleases.
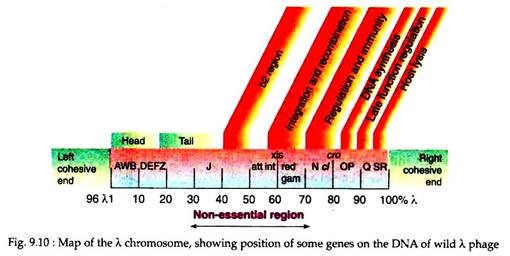
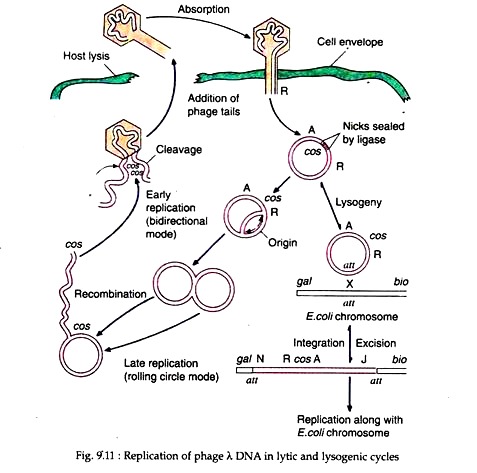
Due to this cohesive properties such sequence is called the cos sequence (Fig. 9.10). Once inside the bacterial cell, the cos sequences base-pair and sealing of the nicks by cellular ligases results in the formation of a double-stranded circular DNA. After that the λ DNA can undergo one of two alternative pathways.
(i) The Lytic Cycle:
In the lytic cycle, the viral DNA replicates, initially bi-directionally, and subsequently by a rolling circle model resulting in linear multi-mers of the virion. The λ multi-mers are snipped at cos sites to produce unit lengths of λ genomes which are packaged within the synthesised coat protein. Some of the λ gene products cause lysis of the host cell wall, allowing the virions to escape and infect new bacteria (Fig. 9.11).
(ii) The Lysogenic Cycle:
The lambda genome possesses a gene att which has a homolog in the E. coli chromosome. Apposition of the two att genes can cause recombination between the λ and E. coli genomes and subsequent integration of the λ DNA within the E. coli chromosome. In this condition the phage DNA is called provirus and the host cell as lysogen.
The phage DNA can remain stably integrated for long periods, but it has the capacity for excision from the host chromosome and entry into the lytic cycle (Fig. 9.11). The genes controlling the lysogenic cycle of the phage are located in a central segment of the λ genome.
However, the decision of the phage to enter either lytic or lysogenic cycle is controlled by two regulatory genes; cl and cro which are mutually antagonistic. In the lytic state, the cro gene dominates, causing the repression of cl.
Whereas, in the lysogenic state, the cl dominates, and suppresses transcription of some λ genes including cro. Normally, the lysogenic state is favoured and the phage genome is replicated along the host chromosomal DNA.
In the DNA genome, λ genes encoding the head, tail proteins and proteins responsible for lytic and lysogenic growths are clustered in discrete regions of the ≈ 50kb viral genome. Genes that are not involved in lytic pathway and that are involved in lysogenic pathway are not essential for the use of the λ phage as a cloning vector. So, these genes may be removed from the viral DNA and are replaced with other DNA fragment of interest. In this way foreign DNA up to ≈ 25 kb can be inserted into the λ genome.
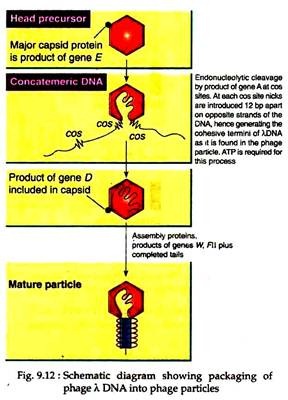
The recombinant λ phages are able to transform E. coli cells at high efficiency. It is achieved by developing an in vitro packaging system which mimicked the way in which wild-type λ DNA is packaged in a protein coat, resulting in high infection efficiency (Fig. 9.12). In this way several important cloning vectors have been developed by modifying the lambda phage genome.
Replacement λ vectors:
The λ genome contains some genes at the central segment that are required for the lysogenic cycle, but not for lytic function. Therefore, replacement of λ vectors can be prepared by replacing this segment with a DNA segment of interest. Using this strategy, foreign DNA up to 23 kb in length can be cloned in such vectors. Such vectors are normally used for making genomic DNA libraries.
Example:
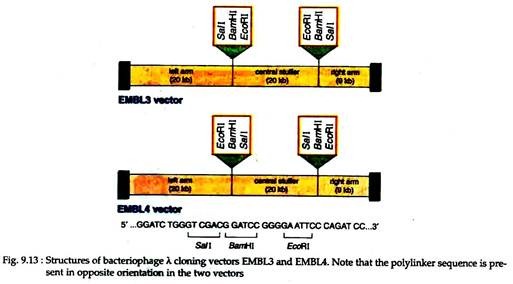
EMBL3 and EMBL4 (Fig. 9.13). These two vectors are so designed that a central non-essential part of about 44kb long can be replaced by a foreign DNA. Cleavage of the phage with appropriate enzymes generates three fragments — left arm, right arms and a central fragment called stuffer. This central part can be replaced by foreign DNA of 20-23 kb long.
Non-recombinant DNA resulting from fusion of left and right arms will give a DNA that is too small for packaging and, therefore, can be automatically selected against recombinant phages. These two vectors have poly-linkers with reverse orders of restriction sites with respect to each other.
Insertion λ vectors:
These vectors are used for making cDNA libraries. Insertion vectors are prepared by modification of the λ genome to permit insertional cloning into the cl gene.
Example:
λgt10 is a 43 kb double stranded DNA for cloning fragments that are only 7 kb long. The insertion of foreign DNA inactivates cl gene and generates a cl– phage. Non-recombinant λgt10 is cl+ and forms cloudy plaques in appropriate E. coli host cells, while recombinant λgt10 is cl– and forms clear plaques in E. coli hosts.
It allows screening of recombinant phages. Further, in the E. coli strain carrying hflA150 mutation (high frequency lysogeny mutation) only cl– phage will form plaque because cl+ will form lysogens and will not undergo lysis to form any plaques. Thus, recombinant λgt10 plaques can be easily selected.
Type # 3. Cosmids as Vectors:
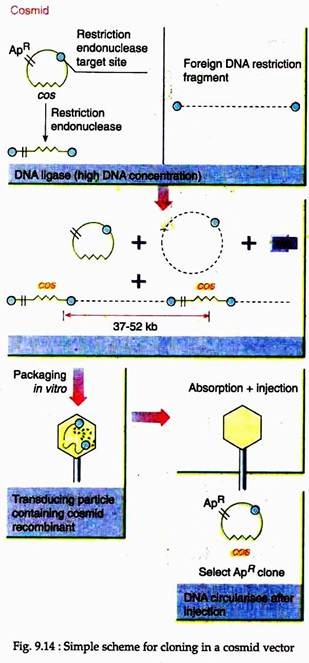
Cosmid vectors are prepared by inserting the cos sequence of a phage into a small plasmid vector, since the cos sites enable the DNA to get packed in lambda particle, cosmids allow the packaging of DNA in phage particle in vitro, thus permitting their selection as well as purification.
Like plasmids, these cosmids perpetuate in bacteria and do not carry the genes for lytic development. The most important advantage of the use of cosmids for cloning is that its high efficiency to produce a complete genomic library of 106 -107 clones from a mere 1 pg of insert DNA. However, the disadvantage is its inability to accept more than 40-50 kbp of DNA (Fig. 9.14).
Type # 4. Phagemids as Vectors:
Phagemid vectors are prepared artificially like cosmid by combining the features of phages with plasmids, as the name suggests. In general, the phagemids possess following characteristics:
(i) A multiple cloning site,
(ii) An inducible lac promoter, and
(iii) An origin of replication (derived from both phage and plasmid).
Example:
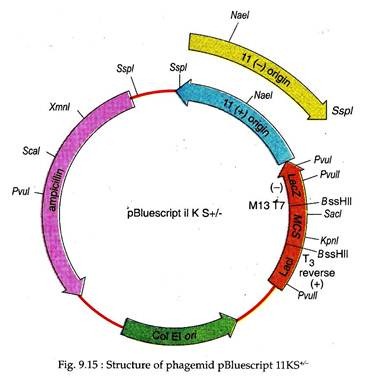
pBluescript IIKS. It is derived from the plasmid pUC19 and is 2961 bp long. The KS designates the orientation of poly-linker in such a way that the transcription of lac Z gene proceeds from the restriction site for Kpnl to Sacl. This artificial vector has a multiple cloning site flanked by T3 and T7 promoters and an inducible lac promoter (Lac I), upstream of lac Z region which complements with E. coli (lac Z–).
It helps in selection of chimeric vector using the criterion of white colonies formed against blue colonies obtained if no foreign DNA is inserted. This vector also contains -fl(+) and fl(–) origin of replication derived from a filamentous phage for recovery of sense(+) and antisense (–) strands of lac Z gene, when host is co-infected with a helper phage; an origin of replication (ColE1 ori) derived from plasmid that is used in absence of helper phage; a gene for ampicillin resistance for antibiotic selection of chimeric phagemid vectors (Fig. 9.15).
Type # 5. Vectors for Cloning Larger DNA Fragments:
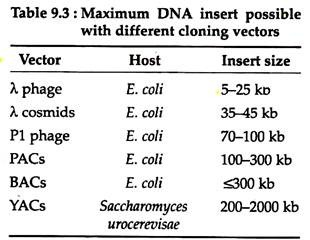
Vectors for cloning large DNA fragments are often required, because human and other mammalian genomes are very large. Recently, a number of such vectors have been developed which are very useful for characterisation and expression of large genes or gene complexes. These vectors have various cloning capacity (Table 9.3).
A. Bacterial Artificial Chromosome (BAC) Vectors:
Most vectors that are used for cloning DNA in bacterial cells contain high to moderate number of replicons. Such vectors yield high number of DNA clones, depending on the replication efficiency of its replicon.
However, the disadvantage of such vectors is that such vectors often show structural instability of the inserts, resulting in deletion or rearrangement of the part of the cloned DNA. Such phenomenon is common in case of DNA inserts of eukaryotic origin where repetitive sequences occur frequently. Therefore, it is very difficult to clone and maintain large DNA in bacterial cells.
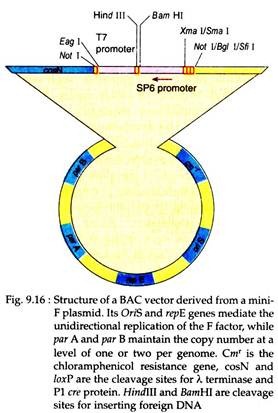
Molecular biologists have developed such vectors which have low copy number of replicons in order to overcome the above limitation. One such vector is prepared from the E. coli fertility plasmid or the F-factor. It contains two genes far A and par B which maintain the copy number of the F-factor at 1-2 per E. coli cell.
This vector can accept large foreign DNA fragment (> 300 kb) and the recombinant vector can be transferred into bacterial cells using electroporation (a method in which cells are exposed to high voltage in order to relax the selective permeability of their plasma membrane). In case of the use of such vectors, however, a low yields of recombinant DNA will be recovered from the host cells (Fig. 9.16).
B. Bacteriophage P1 and P1-derived Artificial Chromosome (PAC) Vectors:
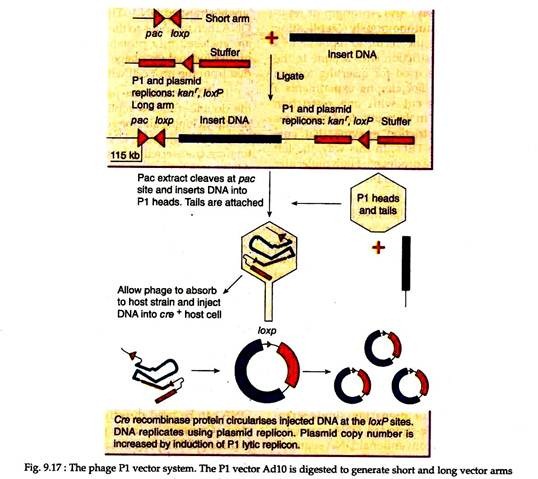
Some bacteriophages have relatively large genomes and thereby can accommodate larger DNA fragments. One such is bacteriophage PI which can package 110-115 kb of linear DNA in the PI protein coat. The P1 cloning vectors are prepared from components of P1 phase that are included in a circular plasmid.
The plasmid vector is cleaved to generate two vector arms to which up to 100 kb of foreign DNA is ligated and packaged into a PI protein coat in vitro.
This recombinant PI phage is then allowed to adsorb to a suitable host, following which the recombinant PI DNA is injected into the cell, circularized and are amplified (Fig. 9.17). Use of another bacteriophage T4 in vitro packaging system with P1 vectors enabled recovery of inserts up to 122 kb in size. Later on, features of P1 and F- factor systems have been combined to develop P1-derived artificial chromosome (PAC) cloning system.
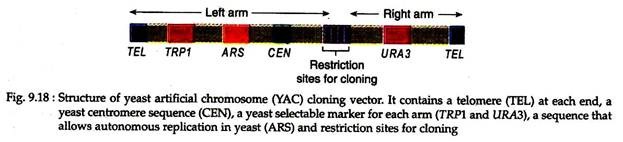
C. Yeast Artificial Chromosomes (YAC):
Construction of yeast artificial chromomes (YACs) provides more advantages over cloning in bacterial cells. It can clone very large DNA fragments.
A YAC has the following features:
(i) A yeast telomere (TEL) at each end.
(ii) A yeast centromere sequence (CEN) allowing regulated segregation during mitosis.
(iii) A selectable marker on each arm for detecting the YAC in yeast (for example, TRP1 and URA3 for tryptophan and uracil independence in trp1 and ura3 mutant strains respectively).
(iv) An origin of replication ARS (autonomously replicating sequence) allows the vector to replicate in a yeast cell,
(v) Restriction sites unique to the YAC that can be used for inserting foreign DNA (Fig. 9.18). For cloning experiments a circular YAC is cut with one restriction enzyme that cuts in the multiple cloning sites and another restriction enzyme that cuts between the two TELs. In this way, the left and right arms are produced. High molecular weight DNA is ligated to the two arms.
The resulting YAC cannot be transfected directly into yeast cells. Instead, yeast cells have to be treated in such a way so as to remove the external cell wall. The resulting yeast spheroplasts can accept exogenous fragments but are osmotically unstable and need to be embedded in agar.
The overall transformation efficiency is very low and the yield of cloned DNA is also low (about one copy per cell). Nevertheless, the capacity to clone large exogenous DNA fragments (up to 2 Mb) has made YACs a vital tool in creating physical maps of large genomes such as the human genome.
Type # 6. Vectors for Preparing Single-Stranded DNA:
Single-stranded DNAs are used as templates for conventional DNA sequencing. These can be prepared by PCR-based methods. Besides these can be obtained using vectors based on certain bacteriophages whose genomes assume a single-stranded DNA at some stages in their life-cycle.
Example:
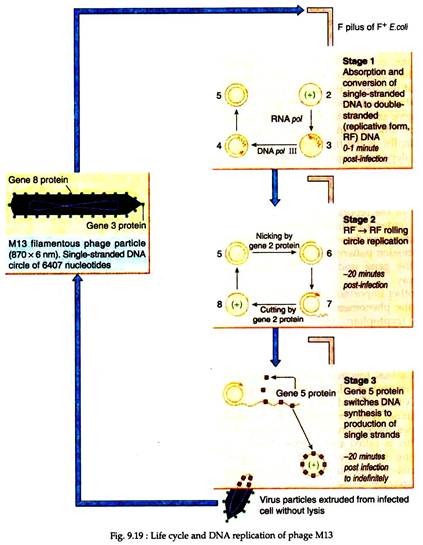
(i) M13 Bacteriophage:
It is a filamentous bacteriophage and its genomes consist of single-stranded circular DNA of about 6.4 kb long, which are closely related to each other in DNA sequence. The genomes are enclosed in a protein coat forming a long filamentous form. Following adsorption to the host E. coli, the genome of the phage is converted from the single-stranded form to a double stranded form (or replicative form) within the host cell.
After sometime, one of the phage genome products switches the DNA synthesis towards the production of single-stranded DNA. These single-stranded DNA then migrates to the cell membrane and are enclosed in a protein coat. In this way, numerous mature phage particles are extruded from the host cell without any lysis.
(ii) M13 Vectors:
M13 vectors are produced based upon the double-stranded replicative form having multiple cloning sites for generation of double- stranded recombinant DNA circles. These vectors are transfected into suitable host E. coli. After some time, the phage particles are harvested and stripped of their protein coat to release the single-stranded recombinant DNA. These DNA can be used as templates in DNA sequencing purpose (Fig. 9.19).
In addition to M13 vectors, phagemid vectors can also be used for preparing single- stranded DNA. In this case, following transformation of a suitable E. coli host with a recombinant phagemid, the host bacterial cells are super infected with a filamentous helper phage, which is required to provide the coat protein.
The released particles from the superinfected cells will be a mixture of both helper phage and recombinant phagemids. However, such mixed population of DNA can be used directly for DNA sequencing. Commonly used phagemid vectors include PEMBL plasmids and the pBluescript family.
Expression vectors:
There are many circumstances where in addition to amplification and propagation of the cloned DNA, it is required to express the gene in some way. In such cases, appropriate expression signals need to be provided by the cloning system. To fulfill this purpose, a large variety of expression cloning vectors has been designed to be used in different host cell systems ranging from bacterial cells to mammalian cells.
An expression vector is a cloning vector containing the regulatory sequences necessary to allow the transcription and translation of a cloned gene or genes. The expression system may be designed simply for investigating expression. In some cases, however, the purpose may be to retrieve large quantities of an expression product, as is the need to generate large quantities of a specific protein that is pharmaceutically important.
Expression vectors are essentially derivatives of the plasmid cloning vectors used in the host. The modifications include the addition of a promoter specific to the host to allow transcription of the cloned gene and if appropriate for the host, also a transcription termination signal. However, if the cloned gene is to be expressed across the prokaryotic- eukaryotic boundary, then the gene may also have to be modified.
Therefore, different mechanisms are used in prokaryotes and eukaryotes for the translation machinery to identify the start codon. For example, expression of a eukaryotic gene in E. coli requires addition of a Shine-Dalgarno sequence at a position upstream of the start codon.
Furthermore, the primary information required about the transcription process involves finding out the size of the transcript, the exact point where the transcript starts and finishes on the gene and how abundant it is at a given point of time within particular tissues or cells.
Finally, as the transcription of gene is largely dependent upon the activity of its promoter, an easily assayable reporter gene is often fused just behind the promoter of the gene being investigated to determine the amount of the reporter gene product being formed under a particular condition. These information, can thus be used to understand the expression patterns of the promoter and hence the gene under investigation in a better way.
Another important issue of gene expression is the phenomenon called codon bias. Except tryptophan and methionine, all amino acids are encoded by two or more codons. In most organisms, not all codons coding for one amino acid are used equally; there is a bias towards using one or more codons instead of others. Four codons viz. GCA, GCC, GCG and GCT code the amino acid alanine.
Now in human, GCC is used four times more than GCG for coding alanine. This is called codon bias. Therefore, depending upon the system to be used, appropriate changes in the coding sequence of the heterologous gene (cloned gene) need to be made to ensure that the correct codon bias is present. This is called codon optimization. It is required for the production of desired protein at maximum level.
It was found that the insecticidal protein ‘cry’, which was obtained from bacteria, was expressed in plants to confer resistance against insect pest; its expression level was very low. The reason was that the codon bias favoured the translation in a bacterial system. However, after codon optimisation, i.e., changing the codon bias from a bacterial system to that of a plant system, the expression of cry gene was improved.
Host Engineering:
Sometimes it is found that the expression of the cloned gene is adversely affected by the host’s genetic makeup. In host cell, presence of protease may degrade the heterologous protein and the recombinogenic activity may bring about the recombination or rearrangement of the introduced heterologous gene. To overcome these problems, some manipulation is done in the host, which is called host engineering.
For example, in E. coli, Ion mutant which is deficient in protease activity is generally used for the expression of the heterologous genes. Again to minimise the chance of recombination of DNA, the recA mutant of E. coli is used. This mutant strain is deficient in RecA protein which is essential for recombination of DNA. Hence, recA mutant show less recombination of heterologous DNA leading to improved expression of cloned genes.
Expression in Bacteria:
For prokaryotic hosts like bacteria, bacterial promoters like those of lac or trp genes would have to be used, which are highly inducible in presence of small amount of certain chemicals like IPTG (isopropylthiogalactoside) in the growth medium.
Modern day vectors for bacterial system use synthetic promoters like tac derived from trp, lac and PL, which combine the high expression properties of several promoters and are capable of giving many-fold higher expression values as compared to individual native promoter. The pET vectors utilize phage T7 promoters to regulate synthesis of cloned gene products. The general strategy for using a pET vector is shown in Fig. 9.20.
Bacteria lack the capacity to process introns, so the heterologous gene to be expressed should be free from introns. Such a gene is then cloned down-stream of the appropriate promoter. In bacteria, often the heterologous gene is expressed as a fusion protein with a bacterial glutathione-s-transferase or maltose-binding-protein.
It helps in the affinity purification of the fusion protein by passing the bacterial extract through a column containing beads of glutathione agarose or amylose resin. The bound fusion protein is then eluted from the column and cleaved with a protease called Factor Xa. It cleaves polypeptide between amino acids arginine and valine.
The vectors contain N- terminal amino acid sequences of bacterial gene, followed by codons for arginine and valine to provide cleavage sites for Factor Xa and then restriction sites for the introduction of the cloned gene to be expressed in the vector system. Thus, this vector system efficiently produces the heterologous protein as a fusion protein, from which the protein of interest can easily be obtained sufficiently.
Expression in Yeast:
Eukaryotic proteins require post-translational modification for functional activity which do not take place in bacterial host. Thus, sometimes, a eukaryotic expression system is required which has more advantages over the prokaryotic vectors. Several species of yeast have been used for this purpose. Most common species include Saccharomyces cerevisiae, Khwyerotnyces lactis, Pichia pastoris etc.
Most yeast expression vectors are prepared by joining a small fragment of DNA from yeast known as the yeast 2 µm fragment to a bacterial plasmid. The 2 µm DNA fragment can serve as the replication origin in yeast and, thus, gives the cloning vector the required replication properties in that system. The expression of the cloned gene takes place under the control of a cloned yeast promoter like that of glyceraldehyde phosphate dehydrogenase.
The system also contains a yeast gene, for example leu, which enables the transformed leu yeast to grow in a medium lacking leucine. It thus helps in the selection of transformed yeast cells. The plasmids are sometimes integrated into the yeast chromosome by homologous recombination, which ensures stable maintenance of the cloned gene. Yeasts can be grown very economically and easily into many media, thus these group of fungi are ideal for obtaining proteins of interest.
Expression in Mammalian cells:
For the expression of cloned genes in mammalian cells, usually several vectors derived from mammalian viruses are used.
These include:
(i) DNA viruses like papoviruses (SV 40 and polyoma) and adenoviruses, but their capacity for foreign DNA is very low, so they cannot be used for expression of many large genes.
(ii) Retroviruses (murine retrovirus) can infect large variety of cell type and can clone large genes, but these are single stranded RNA, so cannot replicate without being integrated. These viruses require replicating cells for expression or for fully differentiated cells which must be present in high concentration.
(iii) Adeno-associated virus is nonpathogenic and overcomes the limitations associated with retrovirus.
(iv) Herpes virus, it is very useful as it is non-integrating and of large size and thus can be used for expression of large genes.
To use the viruses as vectors the pathogenecity genes must be deleted, so that only the replication-associated genes and those for high copy number were retained. The vector system also needs a selection marker, as for example, the neomycin resistance gene which provides resistance against the cytotoxic compound G418.
Such expression vector can be used to express cloned proteins in cultured mammalian cells. Generally, the level of expression by these vector systems is modest but is very important for those proteins which can be correctly folded or processed only in human cells.
Some Special Vectors:
Transposons as vectors:
In Drosophila, the P element is an important transposon, which consists mainly of 31 base pairs terminal repeats enclosing a 3 kilo-base protein coding region. This region codes transposase and a repressor of transposition. However, there occur many deleted P elements which lack transposase or other genes.
In these transposons, gene of interest can be inserted to yield recombinant P elements which can be then microinjected into fertilized eggs along with normal P element (to supply transposase). Thus, inserted gene can be transposed onto an embryonic chromosome.
Plasmid shuttle vectors:
There are vectors that can be used to transform mammalian cells in culture as well as vectors to transform other animal cells, plant cells and yeast cells. These are called shuttle vectors— or cloning vectors that can be introduced into two or more different host organisms. For example, some shuttle vectors can be transformed into and replicate in E. coli and can also be transformed into yeast.
Much such yeast E. coli shuttle vectors have been developed, some of which replicate in high number in the nucleus, some replicate freely as single copies in the nucleus and some integrate into the yeast nuclear chromosome, replicating when that chromosome replicates.
Retriever vectors:
These are another class of vectors which are used to retrieve specific genes from the normal chromosome of an organism like yeast through recombination. These vectors can multiply both in E. coli and yeast. In these vectors, the gene of interest is removed by enzyme to produce gapped vector having flanking sequences.
Through recombination these can acquire lost sequences from the host. Retriever vectors thus are very useful in isolation of genes from yeast for further analysis.