In this article we will discuss about the:- 1. Subject-Matter of T-Cell Receptor 2. Structure of T-Cell Receptor 3. CDRs of T Cell Receptor and their Role 4. Accessory Molecules.
Subject-Matter of T-Cell Receptor:
T lymphocytes or T cells respond only to peptide fragments of protein antigens that are displayed by self-MHC molecules (major histocompatibility complex). T cell receptor or TCR differs from the B cell receptor in two important ways.
First, the T cell receptor is membrane bound and does not appear in a soluble form as the B cell receptor does; second, the T cell receptor is specific not for antigen alone but for antigen combined with a molecule encoded by MHC.
Further, the T cell receptor remains associated on the membrane with a signal-transducing complex CD3 which is non-covalently linked to the receptor to form the TCR complex. The TCR is a clonally distributed receptor, meaning that clones of T cells with different specificities express different TCRs.
ADVERTISEMENTS:
The biochemical signals that are triggered in T cells by antigen recognition are transduced not by the TCR itself but by TCR complex. T cells also express other membrane receptors that do not recognise antigen but participate in responses to antigens; these are collectively called accessory molecules. These molecules deliver signals to the T cell that function in concert with signals from the TCR complex to fully activate the cells.
Antigen recognition by T cells is specific not only for antigen but also for an MHC molecule. T cells were shown to recognise antigen only when presented on the membrane of APC (antigen presenting cell) by a self-MHC molecule. This attribute called self-MHC restriction, distinguishes of antigen recognition by T cells from that by B cells.
Two models were proposed to explain the MHC restriction of T cell receptor. The dual-receptor model proposed that a T cell has two separate receptors, one for antigen and one for class I or class II MHC molecules. The altered self-model proposed that T cell possesses a single receptor capable of recognising foreign antigen, bound to a self-MHC molecule.
However, the elegant experiments by J. Kappler and P. Marrack provided supports for the altered self-model. Unlike the dual-receptor model, in which an antigen and MHC molecule are recognised separately, the altered self-model predicts that a single receptor recognises an alteration in self-MHC molecules induced by their association with foreign antigens.
Structure of T Cell Receptors:
ADVERTISEMENTS:
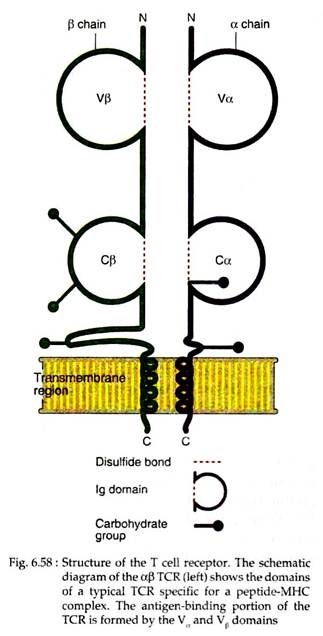
The antigen receptor of MHC restricted CD4+ helper T cells and CD8+ cytotoxic T cells is a heterodimer consisting of two trans-membrane polypeptide chains. These chains are designated as α and β which are covalently linked to each other by disulfide bonds (Fig. 6.58). Another group of TCR, found on a small subset of T cells, has y and δ chains.
In the amino terminal, each a chain and β chain consists of the Ig-like variable domain (V), one constant domain (C), hydrophobic trans-membrane region and a short cytoplasmic region. Thus, the extracellular portion of αβ heterodimer in TCR is structurally similar to the antigen binding fragment (Fab) of an Ig molecule.
The α and β chains of V regions of TCR contain short stretches of amino acids where the hyper-variable or complementarity determining regions (CDRs) are located. Three such CDRs in the α chain are juxtaposed to three similar regions in the β chain to form the part of the TCR that specifically recognises peptide-MHC complexes.
ADVERTISEMENTS:
The P chain V domain contains a fourth hyper-variable region, which does not appear to participate in antigen recognition but is the binding site for microbial products called super antigens. The C regions of both α and β chains continue into short hinge regions, which contain cysteine residues that contribute to a disulfide bond linking the two chains.
The hinge is followed by a hydrophobic trans membrane portion of 21 or 22 amino acids. In a chain, positively charged amino acid residues like lysine and in (3 chain lysine or arginine residues are present in the trans membrane portion. Both α and β chains have carboxyl terminal cytoplasmic tails that are 5 to 12 amino acids long.
Each TCR chain, like Ig heavy and light chains, is encoded by multiple gene segments that undergo somatic rearrangements during the maturation of the T lymphocytes. In α and β chains of the TCR, the third hyper-variable regions (CDR3) are composed of sequences encoded by V and J (joining) gene segments (in the α chain) or V, D (diversity) and J segments (in the β chain).
The CDR3 regions also contain sequences that are not present in the genome but are encoded by different types of nucleotide additions, so-called N regions and P nucleotides. Thus, most of the sequence variability in TCRs is concentrated at CDR3.
CDRs of T Cell Receptor and their Role in MHC-associated Peptide Recognition:
Different experimental studies have established that both α and β chains of TCR form a single hetero-dimeric receptor that is responsible for both antigen specificity and MHC restriction of a T cell. The antigen binding site of the TCR is a flat surface formed by the CDRs of α and β chains.
The TCR contacts the peptide-MHC complex in a diagonal orientation, fitting between the high points of MHC α helices. In general, the CDRl loop of the TCR α and β chains are positioned over the ends of the bound peptide.
The CDR2 loops are over the helices of the MHC molecule and the CDR3 loop is positioned over the centre of the MHC-associated peptide. In fact, the side chain of only one or two amino acid residues of the MHC bound peptide make contact with the TCR. T cells have very remarkable ability to distinguish among diverse antigens on the basis of very few amino acid differences.
The affinity of the TCR for peptide-MHC complex is very low. Such low affinity of specific antigen binding is the likely reason that accessory molecules are needed to stabilise the adhesion of T cells to APCs. The TCR and accessory molecules in the T cell plasma membrane move coordinately with their ligands in the APC membrane to form a transient supra-molecular structure that is referred to as immunological synapse.
This structure regulates the TCR-mediated signal transduction. Virtually all αβ TCR-expressing T cells are MHC restricted and express either the CD4 or the CD8 co-receptors. A small population of T cells also expresses markers that are found on NK (natural killer) cells; these are called NK-T cells.
ADVERTISEMENTS:
T Cell Receptor Complex: TCR CD3:
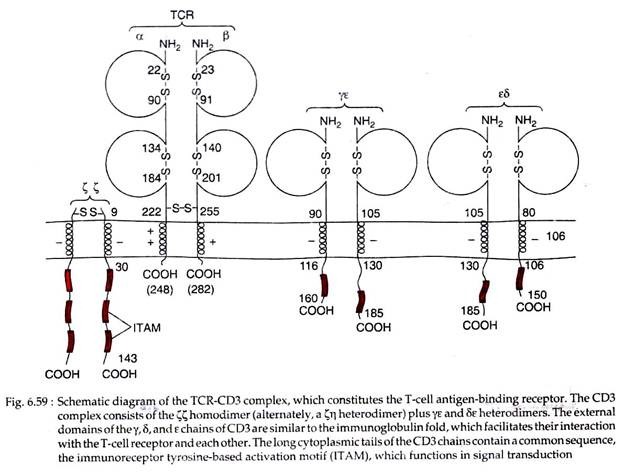
Experiments by J. P. Allison and L. Lanier and others demonstrated that T cell receptor and another protein CD3 are located quite close together in the T cell plasma membrane (within 1-1.5 nm of each other).
The expression of CD3 molecule is required for membrane expression of αβ and yδ T cell receptors; thus each heterodimer forms a complex with CD3 on the T cell membrane. Loss of genes encoding either CD3 or TCR chains results in the loss of the entire molecular complex from the membrane.
CD3 is a complex of five invariant polypeptide chains that associate to form three dimers: a heterodimer of gamma and epsilon chains (yɛ), a homodimer of delta and epsilon chains (δɛ), and a heterodimer of two zeta chains (ϚϚ) or a heterodimer of zeta and eta chain (Ϛƞ) (Fig. 6.59).
The Ϛ and ƞ chains, though encoded by the same gene, may differ in their carboxyl terminal ends because of differences in RNA splicing of the primary transcript. About 90% of the CD3 complexes examined to date have ϚϚ homodimer than heterodimer Ϛƞ as possessed by rest 10% of CD3 complexes.
In CD3 complex, the y, δ and ɛ chains belong to immunoglobulin superfamily, each containing an extracellular domain followed by a trans membrane region and a cytoplasmic domain of more than 40 amino acid residues.
The zeta and eta (Ϛ and ƞ) chains have quite different structure, each with a very short extracellular region of only 9 amino acids, a trans membrane region and a long cytoplasmic tail of 113 amino acids in Ϛ chain and 155 amino acids in ƞ chain.
The trans membrane segment of all CD3 polypeptide chains contains a negatively charged amino acid residue of aspartic acid. Such residues enable the CD3 complex to interact with one or two positively charged amino acid residues in the trans membrane segment of each TCR chain.
The cytoplasmic domains of CD3 Ƴ, ɛ and δ chains contain one copy of a conserved sequence motif called the immuno-receptor tyrosine-based activation motif (ITAM) in each chain. An ITAM contains two copies of the sequence tyrosine-X-X-leucine (X is an unspecified amino acid) separated by six to eight residues.
ITAM plays a central role in signaling by TCR complex. They are also found in Ϛ chain of the TCR complex, Iga and IgP proteins associated with membrane Ig molecules of B cells.
Functions of CD3 complex:
The CD3 and Ϛ chains link antigen recognition by the TCR to the biochemical events that lead to functional activation of the T cells. The earliest intracellular event that occurs in T cells after antigen recognition is the phosphorylation of tyrosine residues within the ITAMs in the cytoplasmic tails of CD3 and Ϛ proteins.
This phosphotyrosines then become the docking sites for adapter proteins and for tyrosine kinase with a kinase called ZAP-70 that binds to the Ϛ chain. Another kinase that also docks at phosphotyrosine is Fyn that binds to CD3. Subsequent activation of these kinases triggers signal transduction pathway that ultimately lead to changes in gene expression in the T cells.
yδ TCR:
In a few T cell populations, a second type of diverse, disulfide linked heterodimer yδ TCR receptor is expressed instead of αβ TCR. This receptor also remains associated with CD3 and Ϛ proteins. (This yδ TCR should not be confused with the y and δ chains of CD3 complex). The TCR y and δ chains contain extracellular V and C domains, short connective or hinge regions, hydrophobic trans-membrane segments and short cytoplasmic tails.
The constituents of these segments are almost similar to those of α and β chains. Furthermore, TCR – mediated signaling events typical of αβ expressing T cells are also observed in yδ T cells. However, majority of yδ T cells do not express CD4 or CD8.
The percentages of yδ TCR expressing T cells vary widely in different tissues and species, but overall, less than 5% of all T cells express this receptor. T cells with yδ TCR are a lineage distinct from the αβ-expressing MHC- restricted T cells.
Many δy T cells present in different organs, may have different V regions, indicating that these subsets may be specific for different ligands. One intriguing feature of yδ T cells is their abundance in epithelial tissue of certain species (e.g., small bowel mucosa of mice and chicken). In human only about 10% of intestinal intra-epithelial T cells express the yδ receptors.
The function of the yδ T cells is not clear. yδ T cells do not recognise MHC-associated peptide antigens and are not MHC restricted. Some can recognise small phosphorylated molecules, alkyl amines or lipids that are commonly found in association with “non-classical” class I MHC-like molecules in mycobacteria and other microbes.
Others may recognise protein or nonprotein antigens that do not require processing or any particular type of APCs for their presentation. Some suggest that they may initiate immune responses to a small number of common microbes that frequently encounter at epithelial boundaries between the host and the external environment.
Accessory Molecules of T Cell Receptor:
T cells express several integral membrane proteins that play important role in antigen recognition and T cell activation. Some of these molecules strengthen the interaction between T cells and antigen presenting cells or target cells; some act in signal transduction and some do both. These protein molecules are often collectively called accessory molecules.
(i) CD4 and CD8:
Mature αβ T cells express either CD4 or CD8 membrane protein, but not both. CD4 and CD8 interact with class II and class I MHC molecules, respectively, when the antigen receptor of T cells specifically recognise peptide-MHC complexes on APCs.
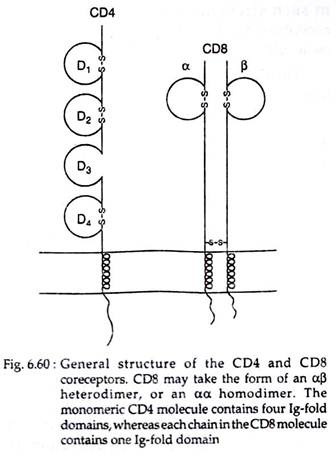
Both CD4 and CD8 are trans membrane glycoprotein members of the Ig superfamily, with similar functions but different structures. CD4 is a 55-kDa monomeric protein that contains four extracellular domains (D1-D4), a hydrophobic trans membrane segment and a long cytoplasmic tail of 38 basic amino acids (Fig. 6.60). It binds through its two N-terminal domains to non-polymorphic β2 domain of the class II MHC molecule.
Most CD8 molecules exist as disulfide-linked heterodimers composed of two related chains called CD8α and CD8β. Both the a and 8 chains have a single extracellular domain, a hydrophobic trans membrane region and a highly basic cytoplasmic tail of about 25 amino acids long (Fig. 6.60).
The extracellular domain of CD8 binds to the non-polymorphic α3 domain of class I MHC .molecules. Some T cells express CD8αα homodimers, but this form appears to function like the more common CD8αβ heterodimers.
Functions of CD4 and CD8:
CD4 binds to class II MHC molecules and is expressed on T cells whose TCRs recognise complexes of peptide and class II MHC molecules. Most CD4+ class II-restricted T cells are cytokine-producing helper cells (TH cells) and function in host defence against extracellular microbes. CD8 binds to class I MHC molecules and is expressed on T cells whose TCRs recognise complexes of peptide and class I MHC molecules.
Most CD8+ class I-restricted T cells are CTLs (cytotoxic T lymphocytes) which serve to eradicate infections by intracellular microbes. However, in humans, some CD4+ T cells may function as CTLs, but even these are class II restricted. Thus, expression of CD4 or CDS determines the MHC restriction of the T cells and not their functional capabilities.
CD4 and CD8 participate in the early signal transduction events that occur after T cell recognition of peptide MHC complexes on APCs. This signal transduction is mediated by a T cell specific Src family tyrosine kinase called Lak that is non-covalently but tightly associated with cytoplasmic tails of both CD4 and CD8. This kinase is also required for T cell maturation and activation.
CD4 and CDS promote the adhesion of MHC-restricted T cells to APCs or target cells expressing peptide MHC complexes. However, in such strengthening function, both co-receptors need the help of other accessory molecules.
The CD4 act as a receptor for the human immunodeficiency virus.
(ii) CD28 and CTLA-4:
CD28 is a homodimer membrane protein that is expressed on more than 90% of CD4+ T cells and on 50% of CD8+ T cells in humans. Binding of B7 molecules on APCs to CD28 delivers signals to the T cells that induce the expression of anti-apoptotic proteins, stimulate production of growth factors and other cytokines and thus promote T cell proliferation and differentiation. Thus CD28 is the principal receptor for delivering signals for T cell activation.
A second receptor for B7 molecule was discovered later and called CTLA-4. It is structurally homologous to CD28 but is expressed on recently activated CD4+ and CD8+ T cells. It inhibits T cell activation by counteracting signals delivered by CD28. Thus, CTLA-4 is involved in terminating T cell responses.
(iii) CD45, CD2:
CD45, a cell surface glycoprotein is believed to play a role in T cell activation. Various forms of CD45 are expressed on immature and mature leucocytes like T and B cells, thymocytes, mononuclear phagocytes and polymorphonuclear leucocytes.
CD2 is a glycoprotein present on more than 90% of mature T cells, 50% of thymocytes and on NK cells. It functions both as an intercellular adhesion molecule and as a signal transducer.
(iv) Adhesion molecules:
Some accessory molecules of T cells function as intercellular adhesion molecules and play important roles in the interactions of T cells with APCs. These molecules also help in the migration of T cells to sites of infection and inflammation. The major adhesion molecules of T cells include integrins, selectins and CD44.
The major functions of integrins are to mediate adhesion of T cells to APCs, endothelial cells and extracellular matrix proteins. The T cell selecting mediates the migration of naive T cells into lymph nodes, where antigens are concentrated and immune responses are initiated.
Selectins also help in the migration of effector and memory T cells to sites of inflammation. CD44 is responsible for the retention of T cells in extravascular tissues at sites of infection. It also causes rapid binding of activated and memory T cells to endothelium at sites of inflammation and in mucosal tissues.