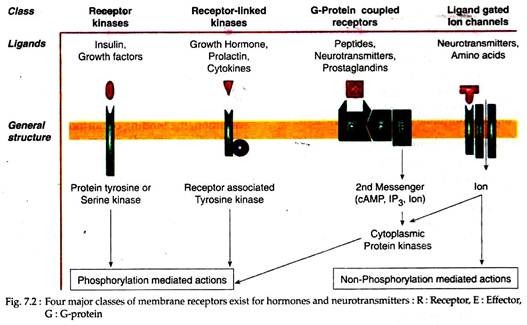
Membrane Receptor # 1. Receptor Kinase:
These membrane receptors contain the effector activity, such as tyrosine or serine kinase activity as an intrinsic part of the structure. The best characterised of these receptors are the protein kinase, as typified by the receptors for insulin, IGF-1, epidermal growth factor (EGF), and platelet- derived growth factor (PDGF) receptors.
Each of these receptors contains an extracellular ligand-binding domain, a single trans-membrane spanning domain, and an intracellular kinase domain. Some, such as the insulin and IGF-1 receptors, are linked by disulfide bonds to form dimers.
Membrane Receptor # 2. Receptor-Linked Kinases:
The membrane receptors for GH, prolactin, granulocyte-macrophage colony stimulating factor (GM-CSF), cytokines (IL-2, IL-4, IL-6 and IL-7), leptin are similar in structure to the receptor kinases and contain a single trans-membrane spanning domain. Although they have no intrinsic enzymatic activity, they interact with other membrane or cytoplasmic proteins that possess tyrosine kinase activity.
ADVERTISEMENTS:
One of such best characterized receptor is Janus kinase (JAK) family of kinases. Several member of this receptor family may also be expressed as circulating non-membrane associated forms, as a result of alternative splicing of an exon encoding the membrane-spanning domain. These circulating receptor forms act as serum-binding proteins for these ligands.
Membrane Receptor # 3. G-Protein Coupled Receptors:
The largest family of membrane receptors uses G-proteins to couple to specific intracellular effector systems, such as the adenylate cyclase or phosphatidyl inositol turnover pathways. Its members include α and β adrenergic, dopaminergic, serotoninergic, muscarinic cholinergic and peptidergic receptors and the receptor for sensing extracellular calcium ions.
These receptors characteristically contain seven trans-membrane domains and like most membrane receptors, have one or more sites of extracellular glycosylation. Each trans-membrane domain consists of 20 to 30 hydrophobic amino acid residues in an alpha-helical structure and is long enough to span the lipid membrane bilayer.
The seven trans-membrane domains are connected by three extracellular and three intracellular hydrophilic loops. Most possess a cytoplasmic tail that contains potential serine phosphorylation sites that may be target for regulation of receptor activity. Seven trans-membrane receptors for peptide hormones usually have a large NH2– terminal extension that participates in ligand binding.
Membrane Receptor # 4. Ligand-Gated Ion Channels:
ADVERTISEMENTS:
Another class of receptor represents the ligand-gated ion channels, of which there are two subtypes: one subtype includes the nicotinic acetylcholine, y-amino-butyric acid (GABA), glycine and kainate receptors, all of which possess four trans-membrane spanning domains.
The second subtype includes the ligand-gated ion channels that encode specific conducts for sodium, calcium and potassium cations and are composed of multiple sub-units, each containing six trans-membrane spanning domains that form homo-multi-mers or hetero-multi-mers.
The ability of such four classes of receptors to bind to different ligands and to transduce specific signal resides in structural subtleties of the specific domain. This also depends on the complex pathway of effector interaction. The interaction of ligand (hormone) with its cognate membrane receptor is the first step in transduction of the hormonal signals from outside of the cell to the inside, where it can modulate function.
ADVERTISEMENTS:
The receptor plays two roles in this transduction process:
(1) Binding the extracellular molecule or hormone that is present in minute amount at the cell surface, with both high affinity and specificity,
(2) Relaying the information inherent in this ligand binding event to sites within the cells.
Hormones may be very small (e.g. catecholamine) to large glycoproteins (e.g. TSH); so the binding domain of receptors must vary to accommodate this range of ligands. Likewise, the signal produced by hormone binding is transduced intracellularly via a multiple effector pathway that includes cAMP, cGMP, arachidonic acid, inositol triphosphate, Ca2+, and other ions as second messengers and are produced by enzymes.
For examples adenylate and guanylate cyclases, phospholipase A2 and C and ion channels. In many cases the hormone-receptor complexes do not interface directly with these effectors but rather act via an intermediate modulating signal transducer such as guanine nucleotide-binding proteins (G proteins).
This large number of receptors and effector components produce intracellular signals that are receptor specific and usually result in signal amplification and divergence into multiple secondary and tertiary effects, and may allow for interaction between related pathways.
Signal transduction: Second messenger concept on hormone action:
“Cell signaling” conveys the concept that the cell is responding to a stimulus from its environment by relaying information to the internal compartment of the cell. Signal transduction is the ability of a cell to convert an external stimulus to an appropriate cellular response.
ADVERTISEMENTS:
Most of the hormones exert their effects on cellular metabolism by binding to receptor on the cell surface and activating a membrane-associated enzyme that in turn elaborates an intracellular second messenger.
This second messenger subsequently mediates the various biological effects of the hormone. Thus, a signal transduction process is reflected in hormone receptor interaction, where the nature of the stimulus received by the cell-surface receptor is entirely different from the signal that is released to the cell interior.
In this regard, Sutherland and co-workers proposed the “second-messenger concept of hormone action”. According to this concept, the molecule that is secreted into the bloodstream, where it is free to interact with membrane receptors on target cells is the first messenger of intercellular communication.
The hormones or other such ligands thus, act as first messengers. The intracellular molecules that are generated following the binding of first messenger to the membrane receptors which mediate the hormone’s effects on intracellular enzymes, gene expression and even on the cell membrane itself, are called second messenger.
cAMP, cGMP, inositol phosphate, ions like Ca2+ or H+ and enzymes such as some protein kinases act as important second messengers. The molecules, associated with cell membrane bring about (effect) the cellular response by synthesizing the second messenger is called an effector, e.g. adenylate cyclase.
Such model of a hormone binding to its membrane receptor, trans-membrane signal transduction, and generation of one or more intracellular signals is the paradigm on which current concepts of peptide hormone and neurotransmitter action are built.
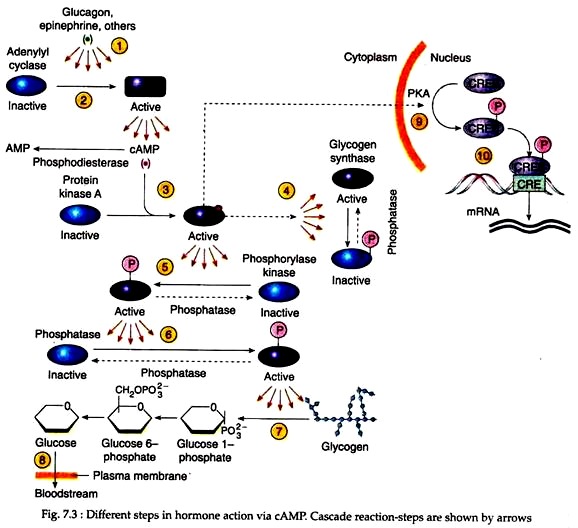
Adrenal catecholamines, glucagon etc. act on the target cells via cAMP that evokes its response by triggering a chain of reactions. This reaction cascade is illustrated in Figure 7.3.
The first step in the reaction cascade occurs as the hormone binds to the receptor; the adenylyl cyclase whose catalytic domains reside at the inner surface of the plasma membrane, become activated and start to catalyze the conversion of ATP into cyclic AMP (step-2).
Each cAMP molecule then binds to an allosteric site on a regulatory subunit of a specific cAMP-dependent protein kinase, called protein kinase A (PKA) (step-3). Cyclic AMP binding causes the dissociation of the inhibitory subunits releasing the catalytic subunits in their active form.
The target substrates of PKA, for example, in liver cells include two enzymes that play a pivotal role in glucose metabolism – glycogen synthase and phosphorylase kinase (Steps 4 and 5). These enzymes subsequently get activated and catalyse different steps in glucose metabolism (see steps 6-8).
It should be noted that binding of a single hormone molecule at the cell surface is able to activate a number of adenylyl cyclase molecules, each of which is able to produce a large number of cAMP messengers in a short period of time.
Each cAMP molecule is then able to activate a single PKA molecule, which is able to phosphorylate a large number of phosphorylase kinase molecules. Thus, in such reaction processes, a great amplification occurs in each step. Therefore, it is referred to as cascade reaction.
Other aspects of cAMP signal transduction pathways:
Cyclic AMP though exerts its effect mostly in cytoplasm, the nucleus and genes of the target cell may also participate in cAMP responses. Some activated PKA molecules may translocate into the nucleus where they phosphorylate key nuclear proteins (step 9), most notably a transcription factor called CREB (cAMP response element- binding protein).
The latter then binds to sites on the DNA (Step 10) containing a particular nucleotide sequence (TGACGTCA) known as the cAMP-regulated enhancer (CRE). CREs then controls the expression of genes that play a role in the response to cAMP.
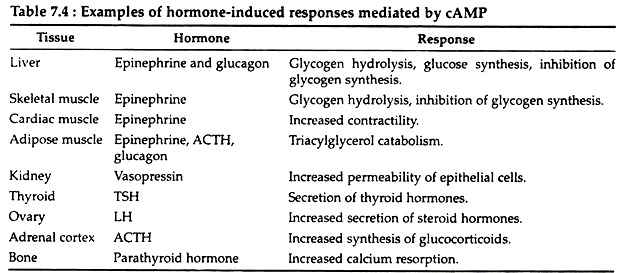
For example, in liver cells several enzymes involved in gluconeogenesis are encoded by genes that contain nearby CREs. Cyclic AMP is produced in many different cells in response to a wide variety of hormones. Few responses mediated by cAMP in mammalian cells are listed below (Table 7.4).
G protein-coupled signal transductions:
Receptors for polypeptide hormones, prostaglandins and neurotransmitters that have seven-membrane-spanning helices are coupled with a heterotrimeric G protein. Transmission of signal from such receptors to effector is accompanied by a third component of the transducer system—the G proteins.
These proteins are so referred as because they bind guanine nucleotides, G protein either GDP or GTP. They are heterotrimeric as all of them consist of three different polypeptide subunits, called α, β and y. Each G protein can exist in two states: an active state, in which the α subunit of the G protein is bound to a GTP molecule or an inactive state in which the α subunit is bound to GDP molecule.
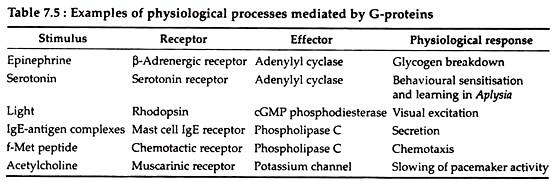
Heterotrimeric G proteins cycle back and forth between these two states depending upon prevailing conditions. A list of some of the ligands that operate via G protein-coupled pathways and their effectors through which they act are mentioned in Table 7.5. The following steps of event occur in all cases of G-protein coupled signal transduction.
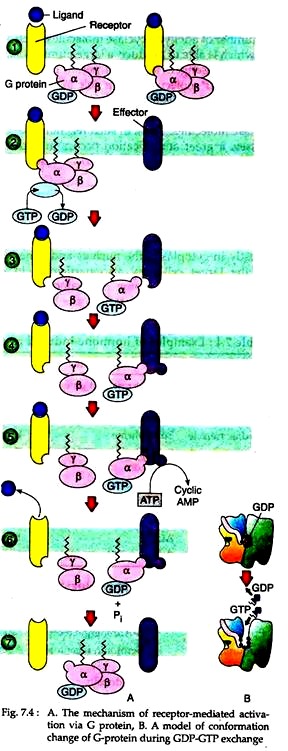
(1) The binding of the ligand to the receptor causes a change in the conformation of the receptor that increases its affinity for the G-protein. As a result, the ligand-bound receptor binds to the G protein on the inner surface of the cell membrane, forming a receptor G-protein complex (Step 1) (Fig. 7.4A).
Interaction with the receptor causes the α subunit of the G-protein to release its bound GDP and bind a GTP replacement (Step 2), which switches the G-protein into the active state (Fig. 7.4B).
(2) The exchange of guanine nucleotides changes the conformation of the Gα subunit, causing it to dissociate from the receptor and form the other two subunits of the G-protein, which remain together as a Gβy complex (Step 3).
Each such dissociated Gα subunit with attached GTP is free to activate an effector molecule, such as adenylyl cyclase, which puts the second messenger system into operation (Step 4). As long as Gα-GTP remains bound to adenylyl cyclase, the enzyme continues to catalyze cAMP from ATP. The cAMP then mediates its second messenger- activities.
(3) The dissociated Gα subunit has its own catalytic activity. It acts as a GTPase, which hydrolyses the bound GTP, forming bound GDP (Step 6). Since GTP hydrolysis shuts off the subunit’s ability to activate additional effector molecules, the α subunit is thus able to inactivate itself. Once the GTP is hydrolyzed, the Gα-GDP can re-associate with the Gβy subunits to reform the trimeric complex and return the system to the resting state (Step 7).
(4) In some cases, the dissociation of the Gα subunit from the receptor, changes the conformation of the receptor so that it loses its affinity for the ligand, which dissociates from its binding site. Thus, the receptor not only transmits a signal inwardly from the ligand to the G-protein, but may also receive a signal that passes outward from the activated G-protein.
Specificity of G-protein coupled responses:
It is now known that a wide variety of substances, including hormones, neurotransmitter, sensory stimuli, are able to use the similar G-protein coupled transduction mechanism for transmitting information across the cell membrane.
Cyclic AMP, if generated, is able to elicit totally different responses in different cells because this second messenger activates a protein kinase that is able to phosphorylate different sets of proteins present in different cell types.
The heterotrimeric G proteins that transmit signals from receptor to effector can also exist in multiple forms. At least 20 different Gα subunits, 5 different Gβ subunits and 7 different Gy subunits have been identified.
Thus, different combinations of specific sub- units are presumed to construct G-proteins that have different properties in their reactions with both receptors and effectors. For example, β-adrenergic receptor is able both to activate adenylyl cyclase and to stimulate the opening of Ca2+ channels, leading to a physiological state in which both cAMP and Ca2+ activated responses can be evoked.
Again some G-proteins act by inhibiting their effectors. Whether a G-protein is stimulatory (Gs protein) or inhibitory (Gi protein) depends on the nature of the α subunit. The same stimulus can activate a Gi protein in one cell and a Gs protein in a different cell.
For example, when epinephrine binds to (3-adrenergic receptor on a liver cell, a Gs protein is activated, which stimulates cAMP production. In contrast, when epinephrine binds to an α-adrenergic receptor on a smooth muscle cell, a Gi protein is activated, which inhibits cAMP production.
Hormones that act via phosphatidylinositol-derived second messenger:
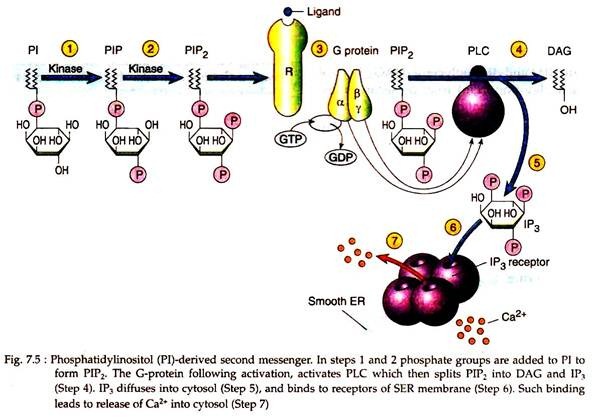
The phosphatidylinositol (PI) is not only a minor component of cellular membrane, but also the precursor for a number of key regulatory molecules. When acetylcholine binds to a smooth muscle, the bound receptor activates a heterotrimeric G-protein (step 3) which in turn activates the effector phospholipase C (PLC) which is situated at the inner surface of the membrane (Fig. 7.5).
Phospholipase C is an enzyme that catalyses a reaction that splits PIP2 (PI biphosphate) into two molecules, inositol 1, 4, 5-triphosphate (IP3) and diacylglycerol (DAG), both of which play important roles as second messengers in cell signalling.
DAG:
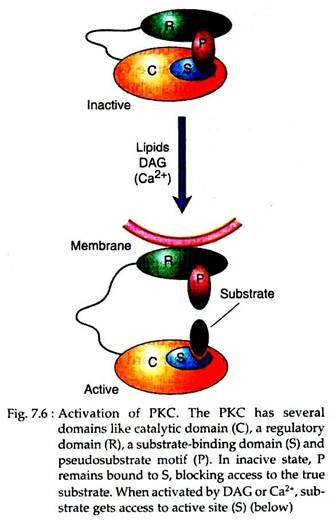
The DAG thus produced may activate an effector (Fig. 7.6), called protein kinase C (PKC) which phosphorylates serine and threonine residues on target proteins. PKC is a family of related enzymes, some of which are activated solely by DAG, whereas others require the presence of Ca2+ ions (hence the C is in the enzyme’s name) as a coactivator. Like PKA, PKC is a multifunctional enzyme.
IP3:
The IP3 that is produced at the membrane following catalytic action of PKC on PIP2, diffuses into the cytoplasm and bind to specific IP3 receptors located at the surface of smooth endoplasmic reticulum. The IP3 receptor of SER also acts as tetrameric Ca2+ channels. Binding of IP3 opens the channel, allowing Ca2+ ions to diffuse into the cytoplasm. Ca2+ ions can also be considered as cellular messengers (Fig. 7.5).
Ca2+ as secondary or tertiary messenger:
Ca2+ ions can act as secondary or tertiary cellular messengers since they can diffuse into the cytoplasm and bind to various target molecules, triggering specific responses. Hormones like vasopressin (pressor effect), catecholamines (α1-effects) and acetylcholine (M1-muscarinic effect) when bind to their receptors, the cytoplasmic Ca2+ concentration of the target cells is enhanced by several ways –
(i) By activation of phospholipase C- poly-phosphoinositol system of signal transduction (as described above).
(ii) By direct inhibition of either Ca2+-ATPase or the Ca2+-Na+ exchanger or the Ca2+-H+-ATPase of plasma membrane so as to decrease the active Ca2+ extrusion from the cells.
(iii) Direct opening of the ligand-gated Ca2+ channels in the membrane so as to increase the down gradient Ca2+-diffusion from the ECF to cytosol. All such mechanisms lead to rise in cytoplasmic Ca2+ concentration.
The rise in Ca2+ level in target cells may modulate the activities of many enzymes such as phospholipases A2 and C, PKC, adenylate and guanylate cyclases, glycogen synthase, myosin light chain kinase, cAMP phosphodiesterase, calmodulin-dependent protein kinase. These may lead to variety of cellular effects including actin-myosin interactions, changes in membrane permeability, exocytosis, endocytosis, cell motility etc.
Calcium ion exerts most of its effects by binding to specific calcium-binding proteins such as calmodulin in non-muscle cells and troponin C in striated muscle cells. Calmodulin has no intrinsic activity of its own, after binding with calcium ion (each molecule of calmodulin has four calcium-binding sites), it is activated and binds to various enzymes or effector molecules causing a change in their activities.
cGMP as second messenger:
Some atriopeptins like atrial natriuretic factor (ANF) that is released from cardiac atria. It is antagonistic to the renal and vascular actions of angiotensin II depends on the signal transduction by guanylate cyclase-cGMP system and on cGMP as second messenger for their actions.
Their binding to specific receptors activates the membrane guanylate cyclase which hydrolyzes cytoplasmic GTP to PPi and cGMP. Cyclic GMP then activates cGMP dependent-protein kinase G (PKG) that is present in smooth muscle and Purkinje cells. PKG then phosphorylates specific protein in the relevant tissue to produce cellular effects like smooth muscle relaxation, vasodilatation, natriuretic and diuresis.
Receptor tyrosine kinases (RTKs): A second major signaling pathway of hormone action:
Mechanism of action of Insulin via RTK:
The target cells of insulin possess insulin receptor at their surface which is more than just a protein that binds a ligand; it is also an enzyme — a protein tyrosine kinase. This receptor being an enzyme adds phosphate groups to specific tyrosine residues of other proteins.
Tyrosine kinases are involved primarily in the control of cell growth and differentiation, rather than the control of intermediary metabolism. Since the insulin receptor has this enzymatic activity it is referred to as receptor tyrosine kinase. Over 50 different RTKs have been identified, which unlike the G-protein coupled receptors, traverse the membrane only once.
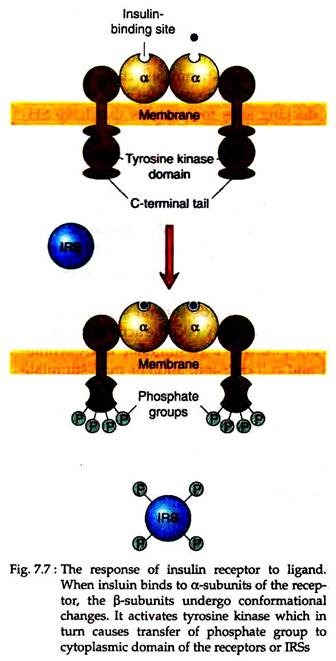
The insulin receptor is a tetrameric protein composed of two α and two β polypeptide chains. The α-chain resides on the extracellular surface of the membrane and contains the insulin-binding sites; whereas the β-chain spans the membrane and transmits the signal across the membrane to its inner surface (Fig. 7.7).
Binding of insulin to the receptor changes the conformation of the receptor, activating its tyrosine kinase. It then adds phosphates to (1) specific tyrosine residues of the other β subunit of the complex, a reaction termed as auto-phosphorylation, (2) a dozen or more strategically located tyrosine residues of at least two protein substrates, called insulin receptor substrates (IRSs).
Phosphorylated IRSs appear to have only one function, which is to bind and activate a variety of “downstream” effectors.
The phosphorylated tyrosine residues in an IRS serve as docking sites for proteins that have SH2 domains, which become activated upon binding the IRS. RTKs phosphorylate only those tyrosines that are present within certain amino acid sequences referred to as “phosphotyrosine motifs”. Proteins having some domains called SH2 domains, show high affinity binding sites for such motifs.
Since a number of different proteins that contain SH2 domains are able to bind to a phosphorylated IRS, several separate signaling pathways may become activated, whereas one pathway may stimulate DNA synthesis and cell division, another may stimulate the movement of glucose transporters to the cell membrane, and yet another may lead to the activation of transcription factors that turn on the expression of a set of insulin-specific genes.
Mechanism of action of other hormones and substances via RTK:
A variety of extracellular agents interacts with RTKs on the surfaces of specific target cells and regulates diverse functions. Among the best studied of these agents are hormones such as growth hormone, growth factors like EGF, FGF, PDGF and cytokines.
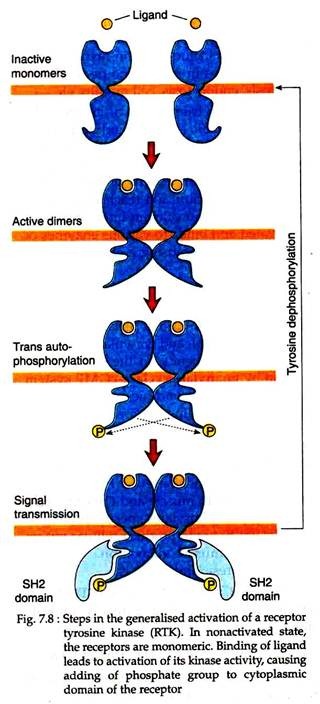
Unlike the insulin receptors as described above, most RTKs are present as monomers in an un-stimulated cell. Following binding of ligand to RTK, they interact with one another to form dimers (Fig. 7.8).
This dimerisation of RTK polypeptide activates their tyrosine kinase activity, causing one subunit of the dimer to phosphorylate a number of tyrosine residues on the cytoplasmic domain of the other subunit of the dimer. The phosphorylation of RTK then sets into motion a chain of reactions in the cell.
Most of the growth factors activate a signaling pathway called MAP kinase (mitogen activated protein kinase) cascade that includes a small monomeric GTP-binding protein called Ras. Like other G-proteins, Ras cycles between an inactive GDP-bound form and an active GTP-bound form. Ras has an intrinsic GTPase activity, which hydrolyses the bound GTP to form bound GDP, thus switching itself off.
When a ligand binds to the RTK, auto-phosphorylation of the cytoplasmic domain of the receptor leads to the recruitment of Ras to the inner surface of the membrane and exchange of its bound GDP with GTP, thus activating G-protein.
Activated Ras has an increased affinity for another protein called Raf, which is recruited to the plasma membrane where it becomes an active protein kinase that initiates an orderly chain of phosphorylation reactions.
The ultimate targets of MAP kinase cascade are transcription factors that stimulate the expression of genes whose products play a key role in the activation of the cell cycle leading to the initiation of DNA synthesis and cell division. The MAP kinase cascade also stimulates protein synthesis.