Steroid hormones exert their action on target cells after binding to specific receptors which are ligand-inducible transcription factors localized primarily within the nucleus. The steroid-receptor complex regulates the synthesis of specific proteins primarily by altering the rate of transcription of specific genes.
The steroid hormone receptors belong to a super-family of so-called nuclear receptors that also includes the receptors for 1, 25- di-hydroxy-vitamin D3, several tissue-specific thyroid receptors and retinoic acid. By phylogenetic analysis, the superfamily can be divided into three subfamilies:
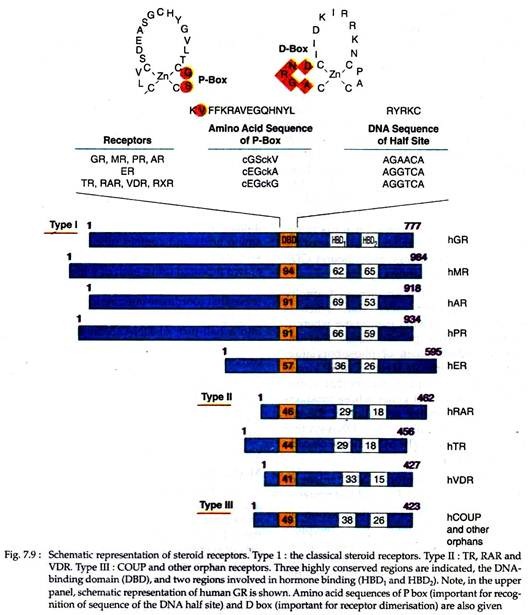
(1) Type I receptors include glucocorticoid receptor (GR), mineralocorticoid receptor (MR), androgen receptor (AR) and estrogen receptor (ER).
ADVERTISEMENTS:
(2) Type II receptors include thyroid receptor (TR), all-trans retinoic acid receptor (RAR), 9-cis retinoic acid receptor (RXR) and 1, 25-di-hydroxy-vitamin D3 receptor (VDR).
(3) Type III receptors include the orphan receptors that have no known ligands. All these receptors have specific domains that are highly conserved structurally and functionally among all members of this superfamily (Fig. 7.9).
All these receptors have a DNA-binding domain (DAB), which is composed of 66-68 amino acids and contain nine perfectly conserved cysteine residues. This domain consists of two repeated units, each of which is folded into a finger-like structure containing four cysteines that coordinate one zinc ion. These DNA binding “fingers” have the capacity to insert into a half-turn of DNA.
ADVERTISEMENTS:
Near the carboxy-terminal end of the receptor molecules are two conserved regions (HBD1 and HBD2) of 42 and 22 amino acids respectively, which comprise the hormone binding domain. The HBD also contains a transcription activation domain, termed as activation function 2 (AF-2), which is essential for ligand dependent activation of transcription by nuclear receptors.
Mechanism of action:
(1) Activation of the receptors:
“Free” glucocorticoid, mineralocorticoid, androgen, and estrogen receptors are known to exist in the cell as monomers associated with a complex of proteins that include phosphoproteins (hsp) and other proeins. In this conformation, the receptor is incapable of binding DNA or regulating gene transcription.
ADVERTISEMENTS:
The binding of hormone to receptors results in the dissociation of receptor from this complex, which forms a homodimer with another receptor molecule. This receptor homodimer has a greatly increased affinity for binding to HRE (Hormone receptor elements in DNA. Upon binding to DNA, the receptor homodimer recruits proteins termed as co-activators which facilitate transcription initiation.
On the other hand, “free” receptors for thyroid hormone, retinoic acid and 1, 25- di-hydroxy-vitamin D3 are not bound to hsps. Rather, they are bound to their HREs either as homodimers or as heterodimers. In their free state, receptors in this subgroup recruit proteins termed as co-repressors that inhibit transcription of their respective target genes.
(2) Regulation of gene expression:
As discussed above, the GR, MR, AR and ER upon binding to DNA, the receptor homo- dimer recruits proteins called co-activators that have histone acetyltransferase activities.
Such co-activators then destabilize the nucleosome structure and cause a local opening of chromatin into a transcriptionally permessive state. Subsequently other transcription factors and RNA polymerase II are recruited and gene transcription is initiated.
The “free” dimeric TR, RAR and VDR interact with a co-repressor complex containing histone deacetylase, which keeps chromatin in a ‘closed’ conformation and silence transcription. The binding of T3 to T3R stabilizes formation of a heterodimer with RXR that causes dissociation of the co-repressor complex and recruitment of a co-activator complex containing histone acetyl-transferases.
Histone acetylation then causes de-stabilisation of the nucleosomal structure, resulting in an opening of the local chromatin structure, into a transcriptionally permissive state that allows assembly of the pre-initiation complex and subsequent recruitment of transcription factors and Pol II. As a result transcription of thyroid hormone-responsive genes is initiated.
With the fall in hormone concentration in the target cells, the hormone receptor complex gets dislodged from the HRE of the DNA and dissociates into the hormone and the receptor. The receptors are immediately inactivated by processes like conformational or ionisation changes, oligomerisation or phosphorylation.