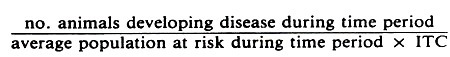In this article we will discuss about the calculation of morbidity rates of animal population.
Morbidity rates describe the level of clinical disease in an animal population and may be crude, cause-specific, attribute-specific (i.e., host characteristic) or a combination of the latter two. Crude rates specify neither disease nor host attributes (e.g., the morbidity rate in feedlot cattle during July was 5%).
Such rates may be made more meaningful by specifying the disease (e.g., the morbidity rate due to pneumonia in feedlot cattle during July was 4%) or attributes of the host (e.g., the morbidity rate in feedlot caves less than 8 months of age during July was 9%) or both.
The extent to which one should make a rate specific depends on the circumstances involved. Morbidity rates also differ depending on whether new cases (incidence) or only existing cases (prevalence) are of interest. Although it is possible to include the number of new and existing cases in the same rate (called period prevalence), it is usually advisable to keep them separate.
ADVERTISEMENTS:
Incidence rates describe the probability, or rapidity, of a new case developing during the stated internal time interval.
The general formula for a crude true incidence rate is:
For example, in a study of calf morbidity the formula for the true morbidity rate per animal month would be:
In most instances, the denominator would be calculated by counting the number of live disease-free calves on the first day of the month, adding this to the number of live disease-free calves on the last day of the month and dividing the sum by 2 (the implied time component being x 1 month) Calves that developed disease during the month would not be at risk at the end of the month and hence should not be included even if they are alive and disease-free at that time.
If detailed calf records were available, the exact denominator could be determined, but often such accuracy is not required. To directly calculate the probability of disease occurrence in a group of animals (e.g., pigs born in July, cattle entering a feedlot in October, dogs whelping in May), one should use the risk form of incidence rate.
For example, the formula for the risk rate of disease in calves born in July would be:
Note that the disease does not have to occur in July. Usually one specifies a reasonable period of risk for the disease in question, say 28 days for most neonatal diseases. Host characteristics (attributes) often have a dramatic effect on the probability of disease events.
ADVERTISEMENTS:
Therefore, most rates are restricted to selected ages or breeds of the species in question; the restrictions apply to both the numerator and the denominator of the rate. An example of an attribute specific rate is a neonatal rate, indicating disease or death within 28 days of birth.
The risk form of rate is frequently used when the event(s) of interest is closely related, temporally, to occurrences such as farrowing (birth), entry to a feedlot, or the start of a racing season; the period of follow up begins at the time of the latter events.
In these instances, the biologic period of risk usually is short relative to the average duration of observation (study period) of individual animals.
For example, since the majority of cases of displaced abomasum (DA) occur within a few weeks of calving, the risk rate formula would be:
In calculating risk rates, the animals in the numerator must belong to the group defined in the denominator.
Of course, if individuals cannot be identified readily, or if new animals are added to the at risk group, the true rate formula:
may be used. Both formulas require that the at risk period for DA be defined. One can convert from the true rate to the risk rate using the formula previously shown. Note that some cows developing DA may not have calved in June and may have contributed little to the denominator.
ADVERTISEMENTS:
Further, some of those calving in June might develop DA in July, but would not be counted in the numerator although they contributed to the denominator. However, in general and particularly in large, stable populations, these discrepancies cancel each other and the rate remains valid. (See Table 3.1 for illustrative calculations.)
For many infectious diseases, animals previously exposed or vaccinated may not be biologically at risk. Thus the rates can be made more accurate if adjustments are made to the denominator for the number of immune animals in the population, and this information should be used if the circumstances allow.
Frequently, however, the number of truly immune animals (as distinct from animals with high-serum titers) is unknown; thus if animals are apparently at risk of the event or disease of interest, they should be counted in the denominator.
In contrast to incidence (a dynamic measure of disease occurrence), the prevalence proportion (also called the point prevalence rate) is a static measure of disease frequency. It is the fraction of the population that is diseased at a point in time.
The general formula for a crude prevalence proportion is:
Note that for a diseased animal to exist, the animal must first develop the disease (a function of incidence); then the disease must persist and the animal must survive (both a function of duration). Thus, in diseases of short duration or with a high case fatality rate, the incidence rate will likely be greater than the prevalence proportion.
Chronic diseases tend to produce prevalence proportions that are greater than the incidence rates. In keeping with common usage, prevalence proportion will be referred to hereafter as prevalence. An approximation that explicitly links incidence rate (IR), prevalence (P) and duration of disease (D) is: P = IR x D. All three quantities must be stated in the same time period (e.g., days).
The terms incidence and prevalence often are used incorrectly, particularly in the reporting of the results of mass serologic or microbiologic testing.
By definition, incidence rates require two tests —one at the start of the period of observation to ensure that the animals did not have the disease, and the second to investigate whether the disease developed during the observation period. Rates based on one test or examination are by definition measuring prevalence (existing cases).
Quite often, rates derived from clinical diagnostic data are treated as incidence rates, as if they were measuring the relative frequency of new cases. However, these rates most often are based on time of diagnosis, not on time of occurrence of the disease. For diseases that may remain subclinical for months or years before becoming clinically apparent, ignoring this difference could lead to inferential errors.
For example, animals born with congenital abnormalities are often thought of as new cases and therefore as incidence cases. However, in order to exist at birth, the abnormality must develop in utero and the fetus must persist (not be resorbed or aborted at an early stage of development).
Variation in the severity of the abnormality, with respect to survivability of the fetus, could drastically alter the number of animals with abnormalities observed at or after birth, with no change in the number of new abnormalities. Thus, congenital abnormalities measure prevalence not incidence.
As demonstrated above, it is quite important to differentiate incidence rates from prevalence proportions.
First, their magnitude may differ greatly, particularly with chronic diseases.
Second, factors associated with acquiring new disease may differ from those associated with having a disease, and only the former are of value for disease prevention.
Finally, knowledge of the time period when the disease was acquired assists in demarcating the time period during which causal factors may have operated and, hence, assists in the identification of these factors.
A subtype of an incidence rate is an attack rate. The latter is used when the period of risk is limited, as in simultaneous exposure of a group of animals to noxious gases or contaminated water or food.
The general formula for an attack rate (AR) is similar to that for the risk form of rate, namely:
Because the biologic period of risk is limited, an attack rate represents the total incidence rate; no new cases would arise from that exposure even if the period of observation were lengthened.
A further modification of morbidity rates, primarily used to study the spread of infectious diseases in defined subgroups (e.g., households) of the population, is the secondary attack rate (SAR), which is calculated as:
Secondary attack rates are usually applied to natural groupings of animals such as pens or farms. They may also be used to evaluate the communicability of diseases of unknown etiology in an attempt to see if infectious agents might be involved. For infectious diseases, the higher the SAR the more contagious the agent.
However, some noninfectious diseases can occur in a manner that may result in a high secondary attack rate. This may occur if there is a variable latent period following a common exposure of individuals within the group, and hence the disease may appear to spread from animal to animal.