In this article we will discuss about the oxygen and carbon dioxide transport in blood of animals.
Requirements of oxygen and removal of carbon dioxide can be met with by simple diffusion only in a very few animals. Gas in solution is also unable to meet the needs. In solution, the plasma can carry only about 0.2 ml oxygen and 0.3 ml carbon dioxide in each 100 ml blood. Presence of haemoglobin in blood increases the capacity by about 100 times in oxygen and 170 times in carbon dioxide.
Oxygen Transport:
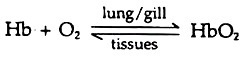
Following concentration gradient oxygen diffuses to the blood from the respiratory membrane. At high oxygen pressure the gas combines with haemoglobin (Hb) and form oxyhaemoglobin (HbO2), an unstable compound. The reaction is reversible.
Each iron atom of haemoglobin molecule can bind one oxygen molecule and when all the four sites are occupied it is called saturated. These are carried to the capillaries and at low oxygen pressure in the tissues the oxygen dissociates from its binding and the haemoglobin gives up all its oxygen. The proportion between haemoglobin and oxyhaemoglobin is regulated by oxygen concentration (pressure).
ADVERTISEMENTS:
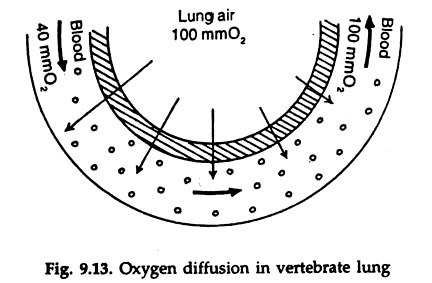
This is known as oxygen dissociation. Haemoglobin is saturated with oxygen at the oxygen partial pressure 100 to 110 mm Hg. No more oxygen is bound by the haemoglobin even at still higher oxygen pressure. At lower pressure oxygen is released and at 30 mm oxygen pressure, half the haemoglobin remains as oxyhaemoglobin. At zero oxygen pressure all oxygen is given up (Fig. 9.13).
Carbon Dioxide Transport:
Only a small part of the carbon dioxide diffusing into the capillaries from the interstitial fluid goes into solution in the blood. Most of the carbon dioxide combines with water to form carbonic acid (H2CO3), which dissociates into hydrogen ions (H+) and bicarbonate ions. The reaction is reversible.
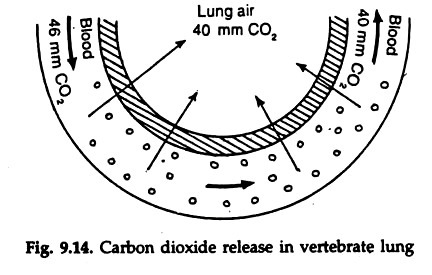
15 to 20 per cent carbon dioxide in the blood binds with the amino group of globin in haemoglobin and form carboamino haemoglobin. By reversible reaction the carbon dioxide is released in the lungs (Fig. 9.14).
Most of the resulting carbon dioxide in the blood is in the form of sodium bicarbonate. HCO3 combines with sodium ions (Na+) in plasma and form sodium bicarbonate (NaHCO3). Carbonic acid and sodium bicarbonate together form a buffer system, which helps in maintaining a constant pH in the blood.
The oxygen dissociation is influenced by carbon dioxide. A higher carbon dioxide concentration leads to release of more oxygen from the haemoglobin at any given oxygen pressure. This is important in removal of carbon dioxide from the tissues.
ADVERTISEMENTS:
With the release of oxygen from haemoglobin in the capillaries carbon dioxide enters from the tissues and the amount of oxygen to be given up from the haemoglobin is increased, leading to additional delivery of oxygen to the tissues.
The total amount of carbon dioxide in venous blood is about 60 ml per 100 ml blood. About 10 ml carbon dioxide is released by reverse reaction in the lungs. The arterial blood contains about 50 ml carbon dioxide per 100 ml blood.
This is known as carbon dioxide tolerance. About 10 per cent carbon dioxide in blood is in solution; about 20 per cent in combination with haemoglobin and about 70 per cent in the form of sodium carbonate (NaHCO3).