In this article we will discuss about:- 1. Introduction to Pancreas 2. Development of Pancreas 3. Location 4. Anatomy 5. Duct System 6. Histology and Cytology 7. Hormones 8. Disorders in Pancreatic Endocrine Function.
Introduction to Pancreas:
The pancreas is the second largest gland associated with the alimentary tract, consisting of exocrine and endocrine tissues. The exocrine part secretes pancreatic juice which is poured into the duodenum via pancreatic duct. Their major role is the processing of ingested foodstuffs so that they become available for absorption.
The endocrine pancreas secretes hormones that are liberated directly into the circulation and modulate cellular nutrition from rate of absorption of foodstuffs to cellular storage or metabolism of nutrients. Dysfunction of endocrine pancreas results in serious disturbances in nutrient homeostasis leading to clinical syndromes referred to as diabetes mellitus.
Development of Pancreas:
In human, the pancreas develops from two duodenal outgrowths arising close to the hepatic diverticulum. Initially each outgrowth consists of a network of anastomosing tubules lined by a single layer of cells. These gradually differentiate into the individual acini that constitute the functional unit of enzyme secretion.
ADVERTISEMENTS:
The precise source of endocrine inlets remains uncertain. Some workers reported that endocrine cells may arise from surrounding acinar cells. Others view is that the islet cells are budded-off from the epithelial lining of the pancreatic ductules.
Location of Pancreas:
The pancreas is a compound gland lying retro-peritoneally on the posterior wall of the abdominal cavity at the level of second and third lumber vertebrae. Commonly it is described as having a head, body and tail. The head is lodged in the concavity of the G-shaped duodenum; the narrow body and tail extend traversely across the posterior wall of the abdomen to the hilus of spleen.
Anatomy of Pancreas:
The pancreas is a pinkish- white organ which in adult measures about 20-25 cm in length and weighs from 100 gm. to 150 gm. It is covered by a thin connective tissue layer that does not form opaque capsule.
Exocrine pancreas:
ADVERTISEMENTS:
The bulk of the pancreas is made of spherical units of cells, the acini. Some acini are small lobules, all acini are bound together by loose connective tissue, through which course the blood vessels, nerves and interlobular ducts.
Endocrine pancreas:
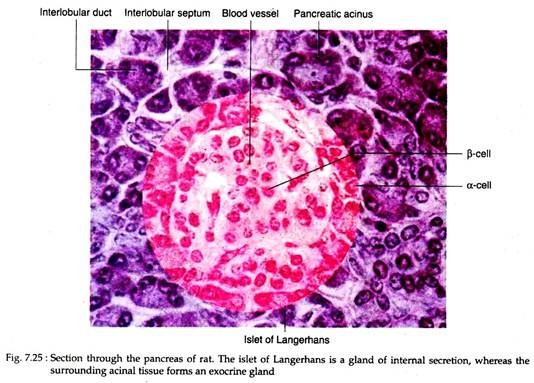
The endocrine tissue of pancreas is segregated in relatively small aggregation of cells forming the islets of Langerhans. These are scattered throughout the gland but are more numerous in the tail than in the body and head of the gland. In human pancreas, over one million of islets are present that constitute only 1-2% of the volume of the gland.
Duct System of Pancreas:
The pancreas is a unique acinar gland in which ducts lined by low cuboidal or squamous cells extend a short distance into the acinus. These so-called centroacinal cells are continuous with the lining epithelium of slender intercalated ducts that drain the acinus.
ADVERTISEMENTS:
These slender tubes then converge to form larger intra-lobular ducts, that are, in turn, tributaries of interlobular ducts in the connective tissue septa between lobules.
These ducts join two main pancreatic ducts. The larger, the duct of Wirsung, begins in the tail, and runs through the length of the gland. In the head, it runs parallel to the common bile duct with which it may have a common opening into the duodenum at the ampulla of Vater. An accessory duct of Santorini lies cranial to the duct of Wirsung and is about 6 cm in length.
The pancreas receives its blood supply from numerous branches of splenic artery and from pancreaticoduodenal branches of hepatic and superior mesenteric arteries. The vessels run through the interlobular septa and give off branches that arborise into capillary networks surrounding the acini and islets.
In exocrine part/the capillary walls have continuous endothelium, whereas the capillaries of the islets are fenestrated. The gland gets its nerve supply from the vegus and splanchnic nerves via the splenic nerve plexus.
Histology and Cytology of Pancreas:
Acini:
The round or spherical or slightly elongated acini consist of 40-50 pyramidal cells around a narrow lumen. The cytoplasm near the base of pyramidal cell harbours closely spaced parallel cisternae of granular endoplasmic reticulum.
The spherical nucleus has a prominent nucleolus and peripheral clumps of heterochromatin. The supra-nuclear Golgi consists of several short stacks of parallel cisternae with numerous associated small vesicles. The apical cytoplasm is filled with large number of dense membrane bound zymogen granules.
Islets of Langerhans:
Each islet is composed of 2,000-3,000 cells which are arranged in irregular cords, separated by a very rich system of capillary vessels or sinusoids (Fig. 7.25). The islets are demarcated from the surrounding acinal tissues by a thin layer of reticular fibers that extend inward to form a delicate investment around the capillaries.
The islets of Langerhans contain four principal cell types, each secreting a different hormone: α-cells or A cells secrete glucagon; β-cells or B cells secrete insulin; δ cells or D cells secrete somatostatin and F cells or PP cells secrete pancreatic polypeptide.
The alpha cells (α-cells) are located mainly at the periphery of the islet but a few are scattered along the capillaries in its interior. The beta cells (β-cells) are predominant cell type and occupy the centre of the islets.
The F cells are widely sattered, very few in numbers and may also occur among the acini as well as in the islets. In these cells, granular endoplasmic reticulum is far less extensive than that of the acini cells. Small vesicles and nascent secretory granules are associated with a juxtanuclear Golgi complex. The most distinctive feature of the islet cells is their secretory granules.
The a-cell granules appear to have homogeneous cores of high electron density surrounded by a narrow outer zone of lower density. The β-cell granules in human pancreas, contain one or more dense crystals in a matrix of low density. The crystals are rectangular or polygonal in section. However, there are considerable interspecific variations in the appearance of β-cell granules.
Hormones of Endocrine Pancreas:
The secretory products of islet cells are given in Table 7.11:
I. Insulin:
Biochemistry:
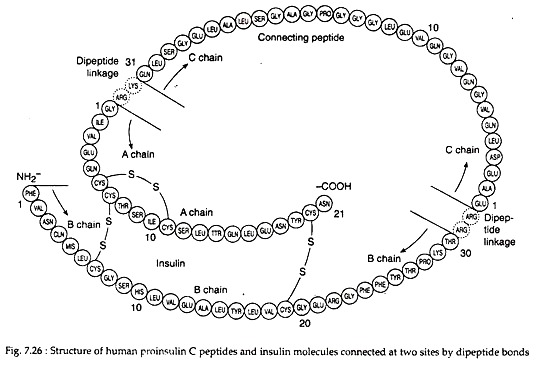
Insulin is a 5.7 kD hetero-dimeric protein composed of 2 peptide chains: an A chain with 21 amino acids and a B chain with 30 amino acids. The chains are connected by 2 disulfide bridges (Fig. 7.26). In addition, there is an intrachain disulfide bridge that links positions 6 and 11 in the A chain.
Biosynthesis:
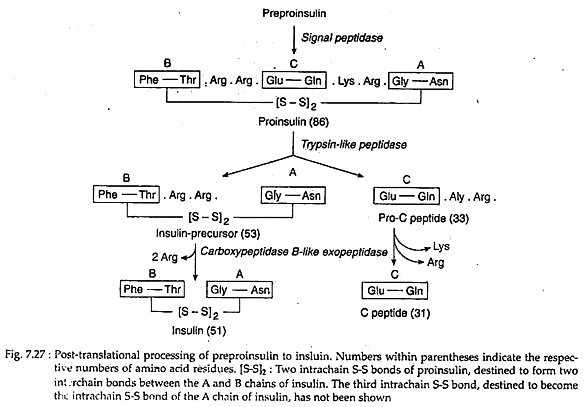
In the cytoplasm of pancreatic β-cells, a long precursor molecule (109 amino acid long) preproinsulin is first translated. Immediately after translation this molecule enters the lumen of RER and from its hydrophobic N terminal, 23-amino acid sequence is hydrolysed away by a signal peptidase to produce 86-amino acid pro-insulin.
It has three intrachain disulfide bonds. In the Golgi, the latter is hydrolysed by a trypsin-like endopeptidase to yield a 33-amino acid pro-C peptide and a 53 amino acid long insulin precursor. The insulin precursor thereby comes to consists of A and B peptide chains, interlinked by two of the S-S bonds of pro-insulin. The third S-S bond of pro-insulin continues as an intrachain S-S bond of the A chain (Fig. 7.27).
An endopepidase eventually hydrolyses the C-terminal peptide bonds in the pre-C peptide and the insulin precursor. This yields two C-terminal basic amino acids from each, changing them respectively to an inactive 31-amino acid C-peptide and the hetero-dimeric protein insulin with a molecular weight of 5.7 kD.
Regulation of secretion :
For insulin secretion there is no pituitary tropic hormonal control. Its secretion is regulated by several factors:
1. Blood glucose level:
The basal concentration of insulin in the blood of fasting human averages 10 µU/ml. Plasma glucose levels below 80-100 mg/dL do not stimulate insulin release. In response to ingested meal, specially glucose, stimulated insulin secretion occurs. When the glucose concentration increases suddenly, an initial short-lived burst of insulin release occurs which is referred to as the early phase.
If the glucose level is held at this level, the insulin release gradually fall-off and then begins to rise again to a steady level (the late phase). This is known as biphasic insulin release. However, sustained levels of high glucose stimulation results- in a reversible desensitisation of the β-cell response to glucose but not to other stimuli.
The actual mechanism behind such glucose-stimulated insulin release in not clearly known. It is known that glucose enters the pancreatic β cells by a specific membrane protein termed as glucose transporter-2. By virtue of its relatively low affinity for glucose, this protein more effectively facilitates transport of glucose during the hyperglycemia after meals than at the lower levels of blood glucose during an overnight fast.
It is also suggested that metabolism of glucose is required in stimulating insulin release.
2. Hormones:
In response to dietary glucose and amino acids, the intestinal mucosa secretes gastric inhibitory polypeptide (GIP) which in turn stimulates insulin secretion from the β cells. Besides this hormone, high blood levels of GH, ovarian steroids, glucocorticoids, vasoactive intestinal polypeptide (VIP), β-adrenergic effect of catecholamine stimulates insulin secretion.
Pancreatic somatostatin and α- effects of catecholamine inhibit insulin secretion.
3. Calcium and cAMP:
Insulin release requires calcium. It has been shown that mature insulin-containing granules in the β cells attach linearly to microtubules that contract after exposure to high intracellular calcium, thereby ejecting the granules. cAMP is another important modulator of insulin release. Glucose is shown to directly induce cAMP formation. Elevations of cAMP, however, will not stimulate insulin release in the absence of glucose.
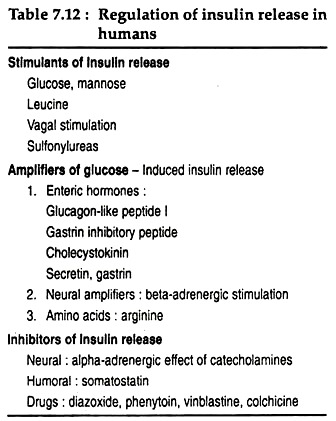
Other factors involved in the regulation of insulin secretion are summarised in Table 7.12. These factors can be classified into 3 groups, viz., factors that stimulate insulin release are direct stimulants, and factors that potentiate the response of the β cells to glucose are amplifiers and inhibitors that inhibit the insulin release.
II. Glucagon:
This polypeptide hormone is synthesised by a-cells of islets of Langerhans.
Biochemistry:
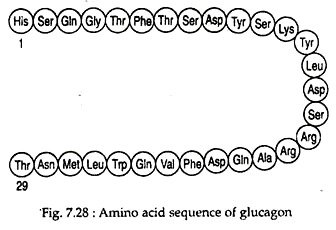
Glucagon is a monomeric polypeptide of about 29 amino acids with a molecular weight of ~3.5 kD. It has no Cysteine residues (Fig. 7.28).
Biosynthesis:
In the α-cells of endocrine pancreatic islets, initially an inactive ~9 kD precursor pro-glucagon is synthesised. This precursor then hydrolysed from both its ends by trypsin-like and carboxypeptidase B-like peptidases of α-cells into glucagon and inactive peptides.
Regulation of secretion:
Like insulin, glucagon has no hypophyseal regulation of its secretion. Its secretion is regulated by different factors:
(1) Glucagon secretion is inhibited by glucose in contrast to the effect of glucose on insulin secretion. It is now known that glucose directly acts on α-cells and its effect is also mediated via release of insulin or somatostatin, both of which are known to inhibit the α-cell directly.
(2) Low blood levels of glucose and free fatty acids stimulate glucagon secretion.
(3) y-amino-butyric acid (GABA) is released by β-cells and it has receptors on a-cell, thus it is presumed that GABA may participate in the inhibition of α-cells during β-cell stimulation.
(4) Other substances that stimulate glucagon secretion include catecholamines, gastrointestinal hormones (cholecystokinins, gastrin, and gastric inhibitory polypeptide), glucocorticoids, sympathetic and parasympathetic impulses, and Ca2+ etc.
(5) High levels of circulating fatty acids are associated with suppression of glucagon release.
III. Somatostatin (SST):
Somatostatin was first identified in the hypothalamus and owes its name to its ability to inhibit release of growth hormone. In addition to D cells of endocrine pancreas, somatostatin has been identified in a number of tissues, like hypothalamus and other areas of brain, the gastrointestinal tract (duodenal mucosa) etc.
Biochemistry:
It is a 14 amino acid cyclic polypeptide with a molecular weight of 1.6 kD.
Biosynthesis:
Somatostatin is first synthesised as a 116 amino acid peptide, pre-pro-somatostatin. From its carboxy-terminus the hormone somatostatin cleaved is cleaved.
Functions:
Somatostatin acts in several ways to restrain the movement of nutrients from the intestinal tract into the circulation. It prolongs gastric emptying time, decreases gastric acid and gastrin production. Pancreatic somatostatin inhibits glucagon and insulin secretion from pancreatic islets by its paracrine action.
IV. Pancreatic polypeptide:
Pancratic polypeptide (PP) is found in F cells located chiefly in islets in the posterior portion of the head of pancreas. It is a 36 amino acid peptide with a molecular weight of 4200. Little is known about its biosynthesis.
In human, PP suppresses secretion of somatostatin from gut and pancreas. It also inhibits gall bladder contraction and pancreatic enzyme secretion. However, the detail physiological functions of the pancreatic polypeptide in human are not delineated.
In dog, an inhibitory control of PP secretion by somatostatin takes place which suggests that SST containing D cells and PP containing F cells control the secretory activities of each other.
Interactions of islet cell hormones:
Normally glucagon secretion is suppressed by relatively small increase in insulin levels, and insulin secretion is stimulated by small increase in the concentration of glucagon. Minute increments in somatostatin suppress the release of both insulin and glucagon, whereas a modest increase of glucagon is required to stimulate somatostatin.
These facts and the non random arrangements of the three major types of islet cells within the normal pancreas have led to the concept of a paracrine system by which the three peptides influence neighbouring cells through the intervening interstitium. However, the major route of within-islet communication is the local circulation which flows from the beta cell rich medulla of the islets to the alpha-cell rich cortex.
This arrangement exposes a-cells to the highest circulating insulin concentration in the body and thus facilitates the action of insulin as an inhibitor of glucagon release. The β-cells, on the other hand receives systemic blood and is, therefore, exposed to lower concentration of glucagon than would be the case if there were direct flow from α-cells to β-cells.
Due to these circulatory arrangements, α and β-cells secrete necessary amounts of glucagon and insulin, respectively, in response to blood hormones and glucose to regulate glucose homeostasis of the body.
Disorders in Pancreatic Endocrine Function:
Insulin and glucagon are the main two hormones that regulate sugar metabolism in the body. A failure of either secretion or action of insulin results in a disease commonly known as diabetes mellitus. An over-secretion of glucagon is the addition factor of this disease. However, diabetes mellitus can also be non-insulin dependent (type II).
A. Insulin-dependent diabetes mellitus (IDDM) or type I diabetes:
It is a catabolic, disorder in which circulating insulin is virtually absent, plasma glucagon is elevated and the pancreatic β-cells fail to respond to all known insulinogenic stimuli. In the absence of insulin, the three main targets of insulin (liver, muscle and fat) not only fail to appropriately take up absorbed nutrients but continue to deliver glucose, amino acids and fatty acids into the bloodstream from their respective storage depots.
Type I diabetes may result from different causes:
Genetics of the disease:
Recent research on entire human genome revealed that not less than 20 independent chromosomal regions are positively linked to this disease. Of these, particularly genes of leukocyte antigen (HLA) DR3, DR4 and genes on the DQ-β chain are strongly associated with diabetes mellitus. However, the pre-dominant antigens associated with type I diabetes may show racial differences.
Autoimmunity of type I diabetes:
It is found that people with certain HLA types are predisposed to development of type I diabetes. On the strength of this evidence, a theory for autoimmune β-cell destruction based on molecular mimicry has been proposed. Here the immune system mistakenly targets pancreatic β-cell proteins that share homologies with certain viral peptides.
Maximum susceptibility to IDDM was found when a certain HLA-DQ gene was present. It expressed on the macrophage surface both a “non-aspartic” amino acid at position 57 on the DQ beta chain and an arginine at position 52 on the DQ alpha chain.
Structural analysis of the DQ protein suggested that this configuration facilitated presentation of certain antigens to the immune system like those peptides as found during the processing of virus coxsackie B4.
More than 85% type I diabetics show a number of autoantibodies. Type I IDDM may occur with other forms of immune endocrinopathy like Hashimoto’s thyroiditis or adrenal insufficiency. Antibodies directed against both cytoplasmic and cell surface determinants on islet cells are present in many type I diabetic subjects.
Rapid development of isletitis and destruction of beta cells occur in pancreas transplanted from unaffected monozygotic twins into the twins with diabetes. All these evidences support the concept that autoimmune mechanisms may contribute significantly to progressive β- cell destruction leading to type I diabetes mellitus.
The most prevalent islet antibodies observed at the onset of IDDM include islet cell antibodies (ICAs); antibodies to insulin, GAD (Ƴ-aminobutyric acid), pro-insulin, immunoglobulins, insulin receptors, carboxypeptidase 11, 37 and 38-kd peptides, islet cell antigen 69 and islet cell antigen 512 etc.
Besides these ICAs, a number of insulin autoantibodies (IAAs), auto-antigens like carboxypeptidase H (the enzyme that converts pro-insulin to insulin), heat shock protein 65, peripherin etc. are also present in type I diabetes patients.
These auto-antigens or autoantibodies are either predominantly humoral or cellular. The type I helper T cells enhance cellular immunity whereas type II helper T cells enhance humoral immunity and both regulate each other through the release of different cytokines. These cytokines in turn induce different natural killer cells (NK cells) and cytotoxic T cells which then destroy the beta cells.
Clinical and physiological changes in IDDM:
The patients of type I diabetes show a variety of symptoms that depend on the age, time of onset and severity of the disease. In general, the patients show weight loss, thirst, blurred vision, postural hypotension, dizziness and weakness.
They also suffer from hyperglycemia, glucosuria, lipidemia, high proneness to kitosis and ketonuria, acidosis, wasting of tissue protein; high urinary levels of urea and creatinine, high plasma levels of 1 K+, polyuria, polydypsia and, in extreme cases, coma or even death may occur due to K+ imbalance.
Hyperglycemia:
Hyperglycemia is a physiological condition when blood sugar level rises above the normal levels (80-100 mg/100 cc). It indicates that the rate of glucose influx into the circulation exceeds that of glucose efflux from the circulation. Insulin is the most important glucose lowering hormone. Reduced insulin secretion in type I diabetes causes different altered glucose metabolism that all lead to hyperglycemia.
These are:
(i) Poor glucose utilisation due to decreased glucose uptake by muscles and adipocytes,
(ii) A fall in insulin-mediated induction of glucokinase causes an indirect decline in glucose diffusion into hepatocytes;
(iii) A lowered rate of glycolysis resulting from the decline in insulin-induced synthesis of hexokinase II and pyruvate kinase enzymes;
(iv) A decline in aerobic metabolism of pyruvate owing to the failure of insulin-mediated dephosphorylation of pyruvate dehydrogenase for its activation. Besides these events there is an enhanced release of glucose from the liver into the blood owing to (i) increased glycogenolysis, and (ii) enhanced gluconeogenesis.
Glucosuria:
Glucosuria is the urinary excretion of glucose. It results when blood sugar level rises above the renal threshold for glucose. The urinary glucose then osmotically retains water in the tubular lumen to increase the urine volume resulting in polyuria. On the other hand, urinary loss of water enhances thirst resulting in polydypsia.
Lipidemia and kitosis:
Pancreatic β-cell destruction or reduced insulin secretion changes the fat metabolism in several ways leading to increased plasma levels of lipids of lipidemia.
Reduced insulin secretion causes:
(i) Increased lipolysis of adipose tissue fat,
(ii) Increased β-oxidation in hepatic mitochondria, and
(iii) Increased ketogenesis. Increased kitogenesis leads to acidosis, ketonuria and even coma due to the depression in brain by ketoacidosis.
Reduced lipogenesis:
In contrast to lipidemia; diabetic individuals show decreased lipogenesis as a result of:
(i) Decreased transfer of citrate from mitochondria to cytoplasm,
(ii) Inhibition of acetyl CoA carboxylase, the rate-limiting enzyme of lipogenesis, and
(iii) Decreased synthesis of insulin-induced enzymes like fatty acid synthase, ATP citrate lyase etc.
Accelerated sorbitol formation:
In diabetes, formation of sorbitol from glucose is enhanced which accumulates in tissues like lens, nerves. This, in turn, causes osmotic influx of water in these tissues and damages its fibrous structure, leading to tissue damages like development of cataract in lens resulting in blurred vision. It may also contribute to peripheral neuropathy and other neuronal disturbances as shown in diabetic individuals.
Protein anabolism:
Diabetes mellitus results in a decrease in those protein synthesis which are regulated by insulin. A drop in insulin level also causes a rise in protein catabolism, wasting of tissues, increase in urea synthesis and high urinary NPN.
K+ imbalance:
Low plasma insulin level reduces the K+ influx into the cells and, consequently, raises the plasma K+ levels which often may cause death.
B. Non-insulin dependent diabetes mellitus (NIDDM) or type II diabetes:
This type II diabetes is a heterogeneous group of milder forms of diabetes resulting from the peripheral insensitivity to insulin. It occurs predominantly in adults and is not linked to HLA markers and with islet cell autoantibodies.
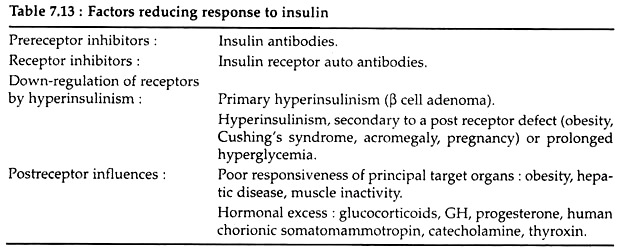
Type II diabetes is defined in negative term; the individuals with type II diabetes are not dependent on exogenous insulin therapy to sustain life – thus the name, non-insulin dependent diabetes mellitus. Different factors that reduce the tissue- responses to insulin are listed in the Table 7.13.
Clinical symptoms and physiological disorders:
The classic symptoms of polyuria, thirst, blurred vision, paresthesia and fatigues are manifestations of hyperglycemia and are, therefore, common to both forms of diabetes. However, most individuals with type II diabetes show obesity and chronic skin infections.
The most characteristic acute catabolic complication of type II NIDDM is nonketotic hyperosmolar coma, a syndrome of extreme hyperglycemia and dehydration. The pathophysiology involves an imbalance between glucose production and its excretion in the urine. Hyperosmolar coma results when the sum of glucose excretion plus utilisation is less than the rate at which glucose enters the extracellular space.
As the extracellular fluid and plasma volumes shrink, the capacity to excrete glucose decreases or disappears as urine volume falls, while hepatic glucose production pours glucose into a shrinking plasma space from which glucose clearance is impaired.
As the plasma glucose level rises, central nervous system dysfunction appears, and the blood glucose level continues to rise, causing extreme hyperglycemia, hyper-osmolality and even death. The mechanisms by which ketoacidosis is suppressed in NIDDM with extreme hyperglycemia is not known. Hyper-osmolality inhibits lipolysis and, presumably, provides less substrate for ketogenesis in the liver.
Other disorders of endocrine pancreas:
Islet cell tumors: Insulinoma:
Tumors in pancreatic β-cells cause excess insulin secretion or insulinoma characterised by severe hypoglycemia. In most cases of insulinoma, clusters of beta cells appear to bud from pancreatic ducts.
Hyperinsulinism in the portal and peripheral circulations, results in low rates of glucose production and rates of glucose utilisation that are inappropriately high relative to the plasma glucose concentration, so that the plasma glucose concentration declines progressively in the post-absorptive state. The most common symptoms of hypoglycemia due to insulinomas include blurred vision, sweating, palpitations or weakness, unconsciousness etc.
Glucagonoma:
Tumors in a-cell may cause glucagonoma syndrome where excess glucagon is produced from α-cells. It is rare tumor that causes disorder characterised by necrolytic migratory erythema, cheilosis, diabetes mellitus, venous thrombosis, weight loss and neuropsychiatry manifestations.
Glucagon excess enhances the hepatic conversion of amino acid nitrogen into urea nitrogen resulting in decreased “blood amino acid levels, weight loss and decreased lean body mass are common features. The glycogenolytic and gluconeogenic effects of glucagon cause diabetes mellitus.
Somatostatinoma:
D-cell pancreatic tumor causes somatostatinoma. Excess somatostatin inhibits diverse endocrine functions in the anterior pituitary, pancreatic islets, gastrointestinal mucosa, thyroid follicle and juxtaglomerular region of the kidney. Suppression of both insulin and glucagon secretion from pancreatic islet may cause mild diabetes mellitus.