The following points highlight the seven major parasites that cause disease in honeybees. The parasites are: 1. Varroa Mite (Yarroasis) 2. Tropilaelaps Mite 3. Tracheal Mite 4. Parasitic Beetles 5. Ants 6. Wasps and Hornets 7. Wax Moths and other Lepidoptera.
Parasite # 1. Varroa Mite (Yarroasis):
This mite (Varroa destructor) is a native parasite of A. cerana throughout Asia. The effect of varroa infestation is to weaken the honey bee colonies and thus decrease honey production. This parasite is found throughout the world, except for Australia and New Zealand South Island.
Varroasis is a brood disease. Occasional removal of A. cerana male brood combs and keeping the hive in healthy condition are the way of prevention of varroasis.
Cause:
ADVERTISEMENTS:
Varroa destructor is quite a large mite species, and can be seen with the naked eye. The shape of the adult female is distinctive. When observed from above, the width of the body is clearly seen to be greater than the length, i.e., about 1.6 x 1.1 mm. The mite is reddish brown in colour and shiny. The body is dorso-ventrally flattened covered with short hair (setae).
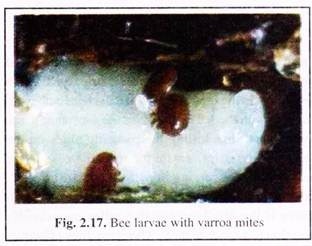
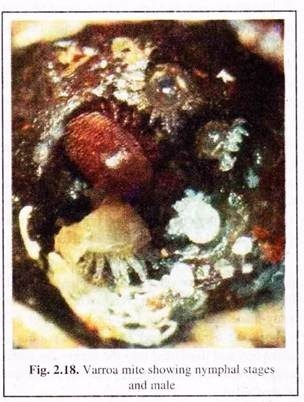
Adult females of V. destructor are found inside brood cells (Fig. 2.17) or walking rapidly on comb surfaces. Adult males, and the immature stages of both sexes (egg, protonymph and deuteronymph), are not commonly seen outside the brood cells. All immature stages of the parasite live inside the brood cells. The immature mites are bright white and the adult females are brown.
Symptoms:
ADVERTISEMENTS:
Varroa causes injuries to honeybees by direct feeding. The adult female pierces the bees’ inter-segmental membrane with their pointed chelicera and sucks the bees’ haemolymph. Varroasis is a brood disease. If more than one parasitic female mite infests the brood cell the brood decays or deformations occur including shortened abdomen or deformed wings.
The bee’s behaviour may be disturbed in orientation or gathering food. The semen production of drones may get considerably reduced. Varroasis is a multi-factorial disease. Normally, the exoskeleton protects the bees from many virus infections. But the mite penetrates this natural barrier, transferring viruses or stimulating the multiplication of viruses with its saliva.
In turn, viruses seem to speed the development of varroasis enhancing the parasite’s virulence. Without treatment the colonies normally die after two to three years, management errors may also cause the collapse of colonies. Colonies destroyed by the varroa mite are often left with only a handful of bees and the queen.
The other bees having died during foraging or having drifted to neighbouring colonies, where the mite population can increase before destroying these colonies also. The presence of adult bees with deformed wings, crawling on comb surfaces or near the hive entrance, usually indicate a late stage of heavy mite infestation. The bees are intoxicated and the mites crawl on the wall (Fig. 2.18).
Control:
The control of V. destructor is one of the most difficult tasks facing apiculturists and beekeepers throughout the world. The mite is a highly successful parasite, whose life history is well synchronized with that of its host.
Two principal approaches to its control are currently available:
(i) Chemical control and
(ii) Hive manipulation techniques, sometimes referred to as biological control.
(i) Chemical Control:
Chemical control is by far the most popular method of varroa control among Asian bee-keepers and elsewhere. Although it creates the risk of honey contamination, the accumulation of residues within the hive and toxic effects to the bees, bee-keepers claim that chemotherapeutic treatment is the quickest and most reliable method of mite suppression.
Among the commonly used mite-control agents are organic acids, ethereal oils, synthetic pyrethroids and amitraz. The application of chemical substances can only be started after honey harvest. This is the only way to avoid residues.
Effectiveness of a chemical substance must be verified in the country side. The organic acids: formic acid, the ethereal oil thymol and the synthetic pyrethroids and amitraz may still be chosen to treat colonies with brood.
ADVERTISEMENTS:
(a) Formic Acid:
Formic acid can kill some of the mites in the sealed brood cells. It is recommended that the formic acid be allowed to evaporate in colonies with sealed brood for at least two to three weeks. In this way, mites emerging from the brood will also be killed.
The formic acid should be introduced into the colony only in the late afternoon to avoid damage to bees and brood. The application can be repeated three to four times at intervals of at least seven days.
(b) Oxalic Acid:
Contrary to formic acid oxalic acid does not act via evaporation but through contact with the bees. Thirty five grams of crystal oxalic acid (dihydrate) is thinned in one litre of sugar water (1:1). When handling crystal acid, special precautions must be taken because of health risks.
Protective spectacles and acid-proof gloves must be used together with an adequate mouth protector. Depending on the size of the colony 20 to 30 ml of the suspension per chamber are dropped into the bee-ways. A repetition of the treatment can lead to damage of the bees.
(c) Lactic Acid:
Lactic acid is clearly better tolerated by bees and does not cause problems in warmer climatic zones. The disadvantage is that every single comb must be extracted to spray the bees with the acid. The dosage applied per comb side is 8 ml of 15 per cent acid. This treatment can be repeated several times at intervals of seven days.
(d) Etheric Oils:
The only etheric oil that is sufficiently effective against varroa mites is thymol. Thymol can be applied as a commercially available ready-made preparation or in crystal form. For this purpose, 0.5 mg thymol per bee-way are put into a gauze bag and deposited into the combs for some weeks. In this way mite emerging from the brood will be eradicated.
(e) Synthetic Chemicals:
Pyrethroids:
Synthetic pyrethroids are contained in Apistan and Bayvarol, which have been developed for application on bees. The plastic strips are fixed in bee-ways in the brood nest. When the bees come into contact with them they transport the substance to the other bees, thus killing the parasitic mites on the bees.
As the strips remain for several weeks in the colony, mites emerging from the brood are affected. Synthetic pyrethroids are highly effective, although there is the disadvantage that mites may rapidly develop resistance to them. Therefore, their effectiveness should be controlled regularly.
Amitraz:
Taktic and Mitac are trade names of products containing amitraz at different concentrations. The recommended dosage for use on honey bee colonies is 1 ml of 12.5 per cent amitraz to 10 litres of water, sprayed lightly on bees, the comb surface of brood frames and hive walls.
The amount of the solution to be sprayed at each application depends on the size of the colony, but is usually within the range of 80 to 250 ml. Amitraz can also be used as a hive fumigant. Fumigation should take place in the evening, when the foragers have returned to the hive.
(ii) Control by Hive Manipulation:
The varroa mite depends on bee brood to complete its development cycle. Since the mite prefers drone brood to worker brood, empty frames are given to the colonies, which will rear drone brood in them. When the cells are sealed, the frames containing the mites get trapped inside the cells, can be removed and destroyed.
The mites can also be trapped in worker- brood frames by using vertical queen-excluders in single-storey hives. The queen is confined between two excluders and allowed to lay eggs in one frame only. Female mites in the colony will be attracted to this brood frame which, when the cells are sealed, is removed from the colony so that the brood cells infested by the parasites can be destroyed.
Parasite # 2. Tropilaelaps Mite:
Modern bee-keeping with Apis mellifera in tropical and sub-tropical Asia frequently encounters problems caused by infestation with Tropilaelaps sp. The mite is a native parasite of the giant honeybee A. dorsata, widely distributed throughout tropical Asia, and whenever A. mellifera is kept within the range of distribution of A. dorsata, mite infestation of the colonies occurs.
Cause:
Tropilaelaps mites are small in size. The adult female mite is light reddish-brown in colour, with an oval-shaped body about 0.96 mm in length and 0.55 mm in width. The mite’s entire body is covered with short setae. A red streak runs longitudinally on the ventral surface of the adult female.
When the mites are present in a honey bee colony in large numbers, they can be observed walking rapidly on the surface of the comb. They are rarely found on adult bees. In all its immature stages, the mite lives within the brood cells of the bees, feeding on the brood’s haemolymph.
Fertilized adult females enter the cells before they are capped to lay their eggs. The stages of development of the mite are as follows: egg, six-legged larva, protonymph, deutonymph, adult. Adult males of Tropilaelaps do not feed, their chelicerae having been modified to transfer sperm. The life cycle of the mite is well synchronized with that of the host bee.
Symptoms:
The abdomen of bees, surviving mite attacks, is reduced in size and they have a shorter life-span than healthy bees. In heavily infested colonies, bees with deformed wings can be observed crawling about the vicinity of the hive entrance and on the comb surfaces.
Inspection of hives severely infested by Tropilaelaps reveals an irregular pattern of sealed and unsealed brood as found with all brood diseases. If mites are present, adult females will be seen walking rapidly out of the cells. To obtain a reasonably accurate estimate of the level of infestation, 100 to 200 cells should be opened and the brood removed with forceps for close inspection.
Control:
Preventing infestation by the Tropilaelaps mite is nearly impossible. As is applicable to other bee diseases, robbery or a too large bee density should be avoided. In recent years, it is reported that the adult female of the mite can survive without bee brood as food, for only up to seven days.
Chemical Control:
Formic acid can be used successfully in its treatment. However, special attention must be paid, in tropical areas, regarding its dosage to avoid damage of the bees. The dosage per comb should not exceed 2 ml in a one-storey Langstrothhive.
Formic acid is placed onto a cloth deposited in the rear section of the hive. Applications of amitraz is very effective, either as a liquid sprayer on the surface of the brood comb and hive walls, or as a hive fumigant, in the same dosage.
The treatment requires three to four applications at four-day intervals. All chemical treatments must be suspended at least eight weeks before the honey flow season arrives and amitraz must not be used in spray form, in the presence of large numbers of honeybee eggs and newly- hatched larvae.
Colony Manipulation Techniques:
Many bee-keepers prefer not to use chemicals to control Tropilaelaps, but to manipulate the brood rearing cycle of their infested colonies in such a way that the mites are deprived of sealed and unsealed brood (their food), for at least three days. During this period, a large proportion of the mite population will starve to death.
There are several means of creating this broodless situation in infested colonies. In smaller apiaries, the bee-keeper can simply remove the brood-comb frames (both sealed and unsealed) from the infested colonies and put them in new hives. Before the new larvae hatch, the hives manipulated in this way will be short of brood for two to three days, which is time enough to starve most of the mites.
The best time of year to carry out these colony- manipulation techniques is during a heavy pollen- flow season, enabling the colonies to rear brood after the period of brood deprivation. Some bee-keepers prefer to combine chemical treatment with the brood-deprivation technique.
In this approach, all sealed brood is removed from the mite-infested colonies, which are then fumigated. The adult female mites, having no capped brood cells in which to hide, are mostly killed by the fumigant, so that only one chemical treatment is required instead of three or four.
Parasite # 3. Tracheal Mite (Acarapidosis):
This mite, Acarapis woodi, infests the tracheal system of adult bees (queens, workers and drones), all are equally susceptible to its attack. Although it was first reported in the Apis mellifera colonies in Europe way back in 1921, its reports from India and Pakistan indicate that the tracheal mite also cause severe losses of A. cerana colonies.
Cause:
A. woodi is a very small mite (0.1 mm) species that lives and breeds within the thoracic tracheae of adult bees. The mite penetrates through the stigma (spiracles) into the first trachea pair of the thorax of a 10-day old honeybee. There it lays eggs at intervals of few days. After the deutonymph stage, male offsprings emerge after around 12 days and females after 13 to 16 days.
Symptoms:
Unfortunately, there are no typically reliable symptoms of infestation in honeybees. Indeed, it has been demonstrated that bees severely infested with the mite can forage normally. Nevertheless, some differences exist with regard to the over-wintering capability of infested and healthy colonies.
Infestation shortens the lifespan of the individual bees, so that severe infestation of colonies causes them to lose strength and thus increases a colony’s susceptibility to winter losses.
The most reliable diagnostic method is laboratory dissection. Samples of 20 or more bees found crawling near the hive and unable to fly are killed, their heads and legs removed and their thoraxes dissected for microscopic examination. If present, the mites are usually found at the end of the first pair of trachea in the thorax.
Control:
Chemotherapeutic measures are widely adopted for mite control. Best results could be achieved with evaporating substances such as formic acid and ethereal oils.
Formic Acid:
Formic acid produces good results by applying it onto a cloth (20 ml of 65 per cent formic acid) four times at intervals of seven days. Treatment should be conducted during the period of low humidity and the temperature should not exceed 30°C.
Menthol or Thymol:
Menthol has a toxic effect on A. woodi in bee colonies. Crystalline menthol (50 g) or thymol (15 g) is placed in a gauze bag on the top of the bars to be kept there for one to two months. External temperatures should be around 21°C; otherwise the menthol vapours will not reach the mites in the trachea.
Parasite # 4. Parasitic Beetles:
There are several different beetles living in honeybee colonies. Most are harmless and feed on pollen or honey.
Small Hive Beetle (SHB):
This beetle is prevalent in Africa and America and there is a risk that the beetle may spread to Asia.
Cause:
The beetle Aethina tumida (order: Coleoptera, family: Nitidulidae) is called the small hive beetle. This beetle lives and multiplies within and outside bee colonies. The beetle produces large cluster of eggs within a bee colony, in fissures and recesses out of reach of the bees.
The larvae of this beetle preferred to live in pollen chamber of honeycombs. The adult larvae leave the hive to pupate in the earth in front of the apiary. The period of development from egg to adult beetle is at least four to five weeks.
Symptoms:
The beetles and their larvae can infest bee brood and honeycombs in and outside the apiary. There, they form eating canals and destroy the cell caps and the honey starts to ferment. The adult beetle is dark brown to black, around 5 mm long and 3 mm wide. Any minor infestation is difficult to recognize because the beetles immediately hide in the dark.
Control:
The best way to protect against an infestation of the small hive beetle is to keep strong colonies and to remove those that are weak from an apiary. The removed honey combs should be centrifugally extracted, one to two days after harvesting the honey. Currently, a successful control is made possible using a preparation named Checkmate.
This product can kill more than 90 per cent of adult beetles and larvae. The beetle can live and multiply outside the beehive, where it seems to prefer rotting fruits (e.g., apples and bananas) as nesting sites. This is why reinvasion is always possible. The beetle is extremely quick moving and can fly, which contributes to its rapid spread among bee colonies and apiaries.
Parasite # 5. Ants:
Ants are among the most common predators of honey bees in tropical and subtropical Asia. They are highly social insects and attack the hives en masse, taking virtually everything in them: dead or alive adult bees, the brood and honey. In addition to this destruction, they can also be a nuisance to bee-keepers and may sometimes cause pain from their bites.
Apiaries of Apis mellifera when attacked by ant become aggressive and difficult to manage; weak colonies will sometimes abscond. Many ant genera and species are reported to cause problems to both traditional bee-keepings with A. cerana and to modern bee-keeping with A. mellifera.
Among the most frequently recorded species of ants are the weaver ant (Oecophylla smaragdina), the black ant (Monomorium indicum), Monomorium destructor, Oligomyrmex sp, Dorylus sp, the fire ants (Solenopsis sp) and Formica sp.
Control: Bee-keepers have found that the most effective method of controlling weaver ants is to search systematically for the ants’ nests in the vicinity of the apiaries and. when found, to destroy them by burning. General precautionary measure is to reduce ant nesting sites that include eliminating brush and rotten wood from the apiary and cutting the grass.
A good general defense against ants in tropical apiaries is to place the hives on stands supported by posts, 30-50 cm high and to coat the posts with used engine oil or grease. Frequent inspection and renewed application of grease are both necessary.
A more reliable method is to place the hive-stand posts in tin or plastic containers filled with either water or oil. Regular cleanup is required to avoid the formation of patches of vegetation on earth that can be crossed by ants. Liquids need to be replenished frequently.
Parasite # 6. Wasps and Hornets:
Nearly all countries in Asia report wasps and hornets as common enemies of their honeybees. Among the most frequently reported enemies are social wasps of the genus Vespa, which are widely distributed throughout the world. Colonies of both A. cerana and A. mellifera are frequently attacked by Vespa. Hornet invasion of A. cerana colonies generally causes the bees to abscond.
Similar behaviour is also reported in weak colonies of A. mellifera. Predation by Vespa sp. on commercial apiaries is generally a seasonal problem. Apiaries situated near the foothills and tropical forests suffer more acutely than those on the plains. During attack an initial hunting phase is observed, during which a few hornets capture and kill slow-flying bees one at a time, usually near the entrance of a weak bee colony’s hive.
Later, a slaughtering phase sets in. Some 20 to 30 hornets attack a weak colony, using their strong jaws to maul the bees and dropping the dead and dying bees to the ground. Finally, when this phase has lasted long enough for the colony under attack to have lost most of its defender workers, the hornets invade the hive itself, the honey and brood nest and the wasps carry away any surplus brood to their nest.
Control:
Due to the large body size the foraging range of Vespa sp can be comparatively of large area around their nests, which have populations of many thousands of individuals. Bee-keepers in Japan sometimes adopt methods such as bait-trapping, trapping at the hive entrance and using protective screens.
It has been seen that the real damage inflicted by hornet attacks on honeybee colonies, occur during the slaughter and occupation phases. Killing hornets in the early stage of predation has the effect of disrupting the hunting phase and preventing the predation process from reaching the more destructive phases. Mass destruction of the colonies is thus prevented or, at the least, minimized.
Parasite # 7. Wax Moths and Other Lepidoptera:
(i) The Greater Wax Moth (Galleria mellonella):
The greater wax moth is often reported to cause damage (Galleriasis) both to honeybee colonies and to bee products in tropical and sub-tropical Asia. Empty combs, rendered wax, comb foundation and bee collected pollen, if not properly stored and left unattended, almost always suffer considerable damage from wax moth infestation.
The wax moth is a major pest of A. cerana, often causing colonies of bees to abscond. In wax moth attacks on colonies, the adult female enters the hive at night, through the entrance or cracks in the walls, deposits her eggs directly onto the combs or in narrow crevices that permit oviposition and offers protection against removal by worker bees. About 50 to 150 eggs are laid in each batch.
They are glued together and adhere firmly to the surface on which they are laid. The newly-hatched Galleria larvae feed on honey and pollen. They then burrow into pollen storage cells or the outer edge of cell walls, later extending their tunnels to the midrib of the comb as they grow. At this stage the developing larvae are quite safe from the worker bees.
As they advance into the combs, they leave behind them a mass of webs and debris. The complete destruction of unattended combs usually ensues within 10-15 days. In addition to stored pollen and comb wax, larvae of the greater wax moth will also attack bee brood when short of food. The development time of Galleria larvae depends on two factors – food availability and temperature.
In tropical climates the larvae require only 18-20 days before spinning cocoons and becoming pupae. When weak colonies are infested, the symptom of galleriasis is frequently observed. The emerging adult worker and drone bees are unable to leave their cells because their bodies have been tied up by silken threads spun by the Galleria larvae.
Control:
There are no easy or inexpensive chemotherapeutic measures for controlling the wax moth in living honeybee colonies once infestation has set in. The only possibility is treatment with Bacillus thuringiensis, in a watery suspension, sprayed onto the combs. The effect on the wax-moths larvae persists for several weeks.
Preventive measures include ensuring that the colonies, whether of A. cerana or A. mellifera, are:
(1) Strong and have adequate food stores;
(2) Adapting the hive space to the strength of the colony;
(3) Reducing the hive entrance, sealing cracks and crevices in hive walls;
(4) Protecting the colonies against pesticide poisoning;
(5) Controlling pests and diseases that might otherwise weaken them; and
(6) Removing any wax and debris accumulated on the bottom boards of the hives. Several measures can be taken to prevent or control wax-moth infestation in stored combs and hive products.
Fumigation is the usual treatment, but new combs should be treated less frequently. Among the most commonly used fumigants are naphthalene, ethylene di-bromide and methyl bromide. All, including para-di-chlorbenzene, are very poisonous to bees and humans and, in addition, lead to residues in honey.
The application of sulphur, however, is inoffensive. Sulphur powder is wrapped in newspaper and burned in a metal container. Liquid sulphur from sprayers can also be used. The development of wax moths can be interrupted for several months if the combs are heated at 48°C for three hours. All treatments should be repeated at intervals depending on the level of infestation.
(ii) The Lesser Wax Moth (Achroia Grisella):
The adult lesser wax moth, Achroia grisella are silver-grey in colour, with a distinct yellow head. The insect is quite small, with a slender body. Normal body lengths of adult female and male are about 13 and 10 mm, respectively. The life-span of the adult female is about seven days, during which she can lay 250 to 300 eggs. Infestation by the lesser wax moth usually occurs in weak honeybee colonies.
The larvae prefer to feed on dark comb, with pollen or brood cells. They are often found on the bottom board among the wax debris. As larvae prefer to form small canals between the bottoms of the brood cells, the brood is lifted. The bees continue constructing cells heading upward leading to the typical scratched comb surface.
Control:
The methods employed in controlling Galleria mellonella are equally effective for the control of Achroia grisella.
Other Lepidoptera:
Other moth species are frequently recorded in association with bees and bee products. The Indian meal moth, Plodia interpunctella, is reported to feed on bee-collected pollen. Moths found dead on the bottom boards of beehives include death’s head or hawk moths (Acherontia atropos), which invade the hives to feed on honey. Bee-keepers generally consider them to be minor pests.