In this article we will discuss about the immune responses of human and other mammals to common parasites.
1. Immune Response to Lyme Disease:
Lyme disease is characterised by some initial red rashes on skin and later arthritic and neurologic symptoms. The disease is caused by the spirochete, Borrelia burgdorferi, transmitted by the vector tick.
Following infection with the spirochete, antibodies to a protein associated with the flagella of B. burgdorferi, can be detected in the host’s serum. But these antibodies do not appear to confer protections against the spirochete rather may contribute to the pathogenesis of lyme disease.
The immune complex consisting of spirochete antigens and host-antibody are thought to result in type III hypersensitive reaction. The complexes can activate the complement system, resulting in direct lytic damage to the joints or vasculature.
ADVERTISEMENTS:
The complement split products like C3a and C5a may induce neutrophils to release lytic enzymes causing tissue damage. It is suggested that Borrelia cell wall contains lipopolysaccharide (LPS) which is an inducer of IL-1 in macrophages. The IL-1 causes the sinovial cells to secrete collagenase and prostaglandins. The released collagenase in a joint could lead to the degradation of collagen and destruction of the joint, the final fate of lyme disease.
Unlike human, the mice reservoir host of Borrelia do not develop lyme disease, mice produce high levels of antibodies to two proteins on the outer surface of spirochete envelope. The humans produce antibodies to one flagellar antigen. The mouce antibodies can mount a protective immune response to them.
2. Immune Responses to Protozoan Parasites:
A number of protozoan parasites are found both in tissues and blood of their vertebrate hosts. As a result of their presence, the host always develops some degree of acquired immunity. This immunity may be either complete or partial.
Further, the immunity may be temporary, i.e., may be maintained only while the parasites are present, a condition called premunition. Again, the immunity may be sterile immunity, i.e., it persists after the complete disappearance of the parasites.
ADVERTISEMENTS:
A. Immune responses to malaria:
Both sterile and premunition immunities can occur in case of human malarias, although neither is usually complete. Further, because of the complex life cycle of Plasmodium spp, (the causative agents of malarias), antibodies produced against one stage of Plasmodium may not be effective against another stage.
ADVERTISEMENTS:
Immunization with antigens derived from sporozoites, merozoites, or gametocytes protect only against one particular stage. Again, immune serum from West African strain was found to be effective against East African strains of P. falciparum, but only against the asexual stages in blood and, hence, is not very effective in preventing secondary infections.
Many pathologic manifestations of malaria may be due to the activation of T cells and macrophages, and production of TNF. The sporozoites of Plasmodium express an antigen circumsporozoite (CS) that mediates their adhesion to hepatocytes. The CS protein has a central zone NANP, which makes up the immunodominant B cell epitope of the protein.
The antibody response to CS protein is dependent on helper T cells. Most CS- specific helper T cells recognise epitopes outside the NANP region and some of these T cells epitopes correspond to the most variable residues of the CS protein.
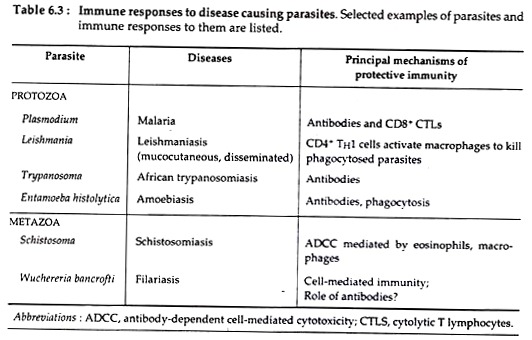
Such findings suggest that variation of the antigens of the surface coat of Plasmodium may have arisen in response to selective pressures imposed by specific T cell responses (see Table 6.3).
CD8+ T cells play an important role in immunity to the hepatic stages of infection. Experimentally the protective effects of CD8+ T cells may be mediated by direct killing of sporozoite-infected hepatocytes or indirectly by the secretion of IFN-y and activation of hepatocytes to produce nitric oxide and other agents that kill parasites.
However, in endemic areas, a low immune response to Plasmodium is usually detected. A number of factors may contribute to such low levels of host immunity. The maturational changes from sporozoite to merozoite to gametocyte allow the organism to keep changing its surface molecules, resulting in continual changes in the antigens seen by the host immune system.
Furthermore, the most accessible stage, the sporozoite, circulates in the blood only for about 30 minutes before it infects liver hepatocytes. It is unlikely that much immune activation can occur in such a short period of time and even when an antibody response does develop to sporozoites.
Plasmodium has evolved a way of overcoming that response by sloughing off the surface CS-antigen coat, thus rendering the antibodies ineffective.
ADVERTISEMENTS:
B. Immune responses to Leishmaniasis:
Leishmaniasis is a complex disease, caused by Leishmania spp that live in the phagosomes of macrophages. People show marked differences in their responses to this protozoan parasites. In fact, dominance of either TH1 or TH2 responses determines disease resistance or susceptibility.
In patients with symptomatic kalaazar, the TH1 responses against L. donovani do not develop, and their macrophages do not secrete IFN-Ƴ or IL-2 in presence of leishmanial antigens. However, these patients regularly have high titres of anti-leishmanial antibodies, that is, their TH2 arm is activated and TH1 arm is down-regulated.
There is an intricate interplay between host immune response and progression of visceral leishmaniasis, and the outcome of this potentially deadly contest is likely influenced by host genotype. Clinical visceral leishmaniasis may not develop for some time, even years, after infection.
Asymptomatic infections may result from early activation of the TH1 arm of the immune response, while kalaazar results from the proliferation of non-protective TH2 cells.
The relationship between host genetic make-up and the immunological response to leishmanial infection has been studied extensively in mice. The gene that controls susceptibility to visceral L. donovani in mice is Lsh gene which has no effect on susceptibility or resistance to cutaneous species of L. major and L. Mexicana.
The severity of cutaneous infections is influenced by another gene, Scl-1, which is non-allelic to Lsh and controls healer and non-healer phenotypes, and a third gene, Scl-2 exerts a ‘no growth’ lesion phenotype that mimics certain clinical pictures in human. In L. major resistant mouse strains, the TH1 arm of the immune response is activated, with production of IFN-Ƴ and a delayed type hypersensitivity reaction.
However, in susceptible mouse strains activation of the TH2 arm stimulates production of IL-4, hyperglobulinemia and elevated IgE levels. The T cells that respond to infection in both cases are those of the lymph node, draining the infection site.
Understanding how these different T- helper responses affect the outcome of infection could provide a more rational approach to the design of effective treatments and successful vaccines for other pathogens.
3. Immune Responses to Helminth Parasites:
Parasitic helminths are responsible for a wide variety of diseases in both human and animals. Unlike protozoans, which are unicellular and often grow within human cells, helminths are large multicellular organisms that reside in human body but do not usually multiply there and are not intracellular pathogens.
Immune responses to Schistosomiasis:
Several species of Schistosoma (S. mansoni, S. janonicum), a trematode worm causes chronic debilitating and sometimes fatal disease Schistosomiasis. Infection occurs through contact with free-swimming larvae, called cercariae, which are released from infected snails. Following contact with human skin, the larvae secrete enzymes which bore into the skin, where they shed their tails and transform into schistosomules.
These schistosomules enter the capillaries and migrate to the lungs, then to liver and finally to the primary site of infection which varies with the species. In the primary sites they mature into male and female adult worms, where male and female produce many spiny eggs.
The eggs produced by the female do not mature into adult in human; instead some of them pass into the faeces or urine and are excreted out to infect more snails. Immune responses by the host against many helminthic infections are mediated by the activation of TH2 cells, which results in production of IgE antibodies and activation of eosinophils.
IgE antibodies bind to the surface of the helminth, eosinophils then attach through Fee receptors, and the eosinophils are activated to secrete granule enzymes that destroy the parasites. Production of IgE antibody and eosinophils are frequently seen in infections by helminths. Actually, helminths first stimulate the TH2 subset of CD4+ helper T cells which secrete IL-4 and IL-5.
IL-4 then stimulates the production of IgE and IL-5 stimulates the development and activation of eosinophils. Eosinophils possess some basic proteins that are more toxic for helminths than the enzymes produced by neutrophils and macrophages. The expulsion of some intestinal nematodes may be due to IL-4 dependent mechanisms that are not well- defined but apparently do not require IgE.
Although the immune responses developed to the infection of schistosomes, in most individuals is not sufficient to eliminate the adult worms, which may survive up to 20 years. Because of their motile nature, the adult worms can evade the localized cellular buildup of immune and inflammatory cells.
The adult worm was also shown to decrease the expression of antigens on its outer membrane and also to enclose itself in a glycolipid and glycoprotein coat derived from the host, making its own antigens. The antigens found on the adult worm are the host’s own ABO blood-group antigens and histocompatibility antigens.
Thus, the host’s immune response is diminished by this covering made of the host’s self-antigens, which must contribute to the lifelong persistence of schistosoma species.
The host’s adaptive immune response to schistosomes can also contribute to many symptoms of schistosomiasis. For example, S. mansoni eggs deposited in the liver stimulate CD4+ T cells, which, in turn, activate macrophages and induce DTH (Delayed type hypersensitive) reactions.
DTH reaction results in the development of fibrosis associated with this chronic cell-mediated immune response leading to disruption of venous blood flow in liver, portal hypertension and cirrhosis.
Host responses to filariasis:
Filariasis is caused by the nematode Wuchereria bancrofti and transmitted by the Culex mosquito vectors. In lymphatic filariasis, lodging of parasites in lymphatic vessels leads to chronic cell-mediated immune reactions and ultimately to fibrosis as happen in case of schistosomiasis. Fibrosis, results in lymphatic obstruction and severe lymphedema, the symptoms of acute stage filariasis.
C. Immune response to African sleeping sickness:
The flagellated protozoan parasite Trypanosoma spp, cause sleeping sickness, a chronic debilitating disease transmitted to human and catties by the bite of the tsetse fly. In the blood stream, trypanosome differentiates into a long, slender form that continues to divide every 4-6 hour.
During later stages of the disease, the parasites infect central nervous system, causing meningoencephalitis and eventually the loss of consciousness.
With the increase in the number of parasites, an effective humoral antibody response develops to the glycoprotein coat, called variant surface glycoprotein (VSG) that covers the trypanosomal surface. These antibodies eliminate most of the parasites from blood stream, by complement mediated lysis and by opsonisation and subsequent phagocytosis.
However, a little number of parasites bearing an antigenically different VSG, escape the initial antibody response. These surviving parasites then begin to proliferate in the blood stream and cause a new wave of parasitemia.
The successive waves of parasitemia reflect an unique mechanism of antigenic shift by which the trypanosomes can evade the host’s immune response to their glycoprotein antigen. From the above discussion it appears that when a pathogen establish an infection in a susceptible host, a series of coordinated events circumvent the host’s immune responses.
In addition to adaptive responses, hosts also exhibit some innate responses to the pathogens that primarily prevent the occurrence of any infection. The first and most important features of host innate immunity is the barrier provided by the epithelial surfaces of the skin and the lining of the gut.
Another major feature of innate immunity is the presence of normal gut flora which can completely inhibit the binding of pathogens to gut epithelium. Phagocytic cells is another highly effective line of innate defence.
Thus both innate and adaptive immune responses to pathogens provide critical defense to hosts. At the same time pathogens also use a variety of strategies to escape destruction by the host’s immune mechanism.