The health of aquaculture organisms, including fishes, is due to a state of physical well-being. Abnormal changes in the body arises from two factors, one for which the aquatic environment is important (dietary deficiency, attack of pathogens and other stresses) and other is due to cold-bloodedness of the fish (fish’s inability to maintain its body temperature).
Nutritional imbalance results in formation of thyroid tumours, anaemia, liver degeneration, visceral granuloma, pigmentation impairment and deficiency symptoms associated with vitamin imbalance.
Physical and environmental stresses play significant role in the maintenance of healthy conditions. Although, many pathogens of aquaculture species are generally found in the aquatic environment, yet their presence may not lead to the occurrence of disease.
Disease results due to interaction between the species, the disease agent and the aquatic environment. The balance between these three major factors determines the state of health of aquatic species. Disturbance in any of these three factors may lead to the disruption of the relationship, resulting in the causation of disease.
Resistance of the Host:
ADVERTISEMENTS:
The resistance or susceptibility of the cultured species to the disease causing agents is governed by its age, exposure experience and physical barriers (such as exoskeleton, skin and mucous membranes). The physical barriers generally restrict the entry of toxic, infectious and parasitic agents.
The physiological defences comprise the white blood corpuscles that engulf pathogens, avoidance mechanisms, detoxification of chemicals from water or diet by the liver, storage of certain metals by the bones and local tissue reactions. The source of fish’s physiological ability to defend itself is the overall nutrition it consumes.
(a) Immune response:
An important means of disease resistance is the immune system of fishes and its specific activity against biological agents such as viruses, bacteria and parasites. The immune system operates through antigen-antibody reactions. The antibodies of fish (teleost) are serum proteins (immunoglobulins) that belong to IgM or IgG class.
ADVERTISEMENTS:
These are functionally classified as agglutinating, precipitating and virus neutralising. By the stimulation from certain antigens (bacteria, virus, etc.) the agglutinating antibodies are formed. These antibodies bind the antigens rendering them less toxic and more vulnerable for easy phagocytosis.
On the other hand, the precipitating antibodies are less important in fish and they generally cause precipitation of soluble antigens which are easily discarded through tests. Virus-neutralising antibodies cling onto the virus particles making them inactive.
In fishes antibody formation is temperature dependent. Lowering of temperature may delay or suppress antibody formation and would thus affect the immune response of that fish. Certain behavioural factors, such as pheromone released in crowded condition, may also suppress antibody formation.
Population with previous exposure (to a specific antigen), will be less susceptible as those on a first encounter. Moreover, young ones are more susceptible to diseases than older ones, due to their fragile defence system.
ADVERTISEMENTS:
(b) Environment:
Environment often plays a vital role in disrupting the balance between the pathogen and the host. Often the culture animals live a healthy normal life in the presence of pathogens. However, during environmental stress the pathogen gets the upper hand and the balance tips in favour of the disease.
At the time of selection of the site for aquaculture the environmental parameters required were adequately considered so that the relevant stress factors are minimum.
The minimum water quality conditions necessary to maintain good fish health are:
Dissolved oxygen — 5 mg/1
Range of pH — 6.7-8.6
Free CO2 — 3 mg/1 or less
Ammonia — 0.02 mg/1 or less
Alkalinity — at least 20 mg/1
ADVERTISEMENTS:
Temperature plays a vital role on a number of other variables in the environment. With increase in temperature solubility of toxic compounds increases creating unfavourable conditions. As has been discussed above antibody formation is temperature dependent. Wound healing is temperature dependent as the repair of dermis is suppressed by low temperature.
Ailments from Environmental Factors are:
(1) Chill and cold weather results in drop of water temperature and the fish shows congestion of gill apparatus and dull appearance.
(2) Alteration in pH of water results in too acidic (pH at or below 5.5) or too alkaline (pH at or above 9) and the fish shows a sign of ailment. Mucus secretion gets enhanced. In too alkaline case, the gills appear burnt and the fins give a rot look. It may result in fatal ailment, if not duly treated.
(3) Depletion in dissolved oxygen content of water results in asphyxia and the fish shows signs of suffocation—mouth gets wide open, gill opercula get raised and the gills spread wide apart.
(4) Constipation and indigestion results in queer movements and listlessness of fish. The fish may remain stationary in mid-water or stand on its head or lie at the bottom on its side. Stomach appears swollen and the scales get erected.
(5) “Egg binding” illness of the females occur during the breeding time. The body of the female fish becomes greatly swollen due to accumulation of ripe eggs that could not be released.
(c) Genetic resistance — As fishes are known to adapt to disease in nature, these traits of resistance can be developed to resist those infections.
However, problems develop in disease-resistant strains of fish (Warren, 1983), such as:
(1) Strains of brook trout, selected for their resistance to furunculosis, were found to have acquired greater susceptibility to bacterial gill diseases during selection.
(2) Strains of steelhead trout, selected for their resistance to bacterial kidney disease (BKD), were also found to be most susceptible to Vibrio anguillarum infections.
Thus, it can be concluded that loss of genetic diversity in a selection process makes it difficult to develop strains of fish that are resistant to several other diseases at the same time.
Even the disease- resistant fish that survives may develop a carrier state, which is harmful if the disease carrier is to be introduced into new areas where the disease would not otherwise occur. Thus, by maintaining a high level of genetic diversity in a stock, the ability increases to withstand the stress of infectious diseases.
Mode of Contraction of Infection:
The contraction of infection is direct
in most parasites and pathogens. The disease causing agents freely swim on to the host or contact it by contiguity, or through a vector (the fish leech transmits Trypano- plasma in cyprinids causing sleeping sickness — Trypanoplasmosis), or infection may be caused through the food medium which is already infected.
For example, transmission of cestode (Ligula) through infected copepods (harbouring the larval stages of the parasite) which is subsequently eaten up by the fish.
Sign of Sickness and Effect on Fish:
Sickness in fishes may vary, but there are some common external symptoms, which are:
(1) Fish becomes listless,
(2) Loss of balance occurs and the ailing fish is unable to maintain its normal position,
(3) The diseased fish tends to lie on its side either floating at the surface or resting at the bottom,
(4) Slimy, grey excretions appear on the skin,
(5) Fading of normal colour occurs and the gills appear pale and
(6) The fins (including tail fin) do not function normally and with vigour.
The parasites of fish extract their nutrition from the host but do not harm it. However, they cause loss of anabolic material and render the host weak.
They, thus, directly or indirectly harm the fish as described below:
(1) Cestodes (Eubothrium) cause mechanical damage as their large-scale multiplication blocks the lumen of the gut.
(2) Trematodes (Diplostomum) cause cataract in the fish eye and impairs vision.
(3) Acanthocephalans (Pomporhynchus) heavily perforates the intestine.
(4) Ligula (cestode) multiplies to such an extent that it constitutes about half the total weight of the host fish. The parasite consumes a substantial part of the fish’s food and thus, retards its growth.
(5) ‘White spot’ and ‘black spot’ cause irritation in the skin and the fish scratches to bleeding.
(6) Argulus from its poison glands discharges toxic substances that may hasten the death of the host fish.
(7) The common aquatic fungus (Saprolegnia parasitica) is a secondary parasite that invariably causes death to the host fish.
Diagnosis of Fish Disease:
The following pathological procedures are undertaken for the diagnosis of various fish diseases:
(1) Assessment offish environment.
(2) Assessment offish health.
A sick fish behaves differently from that of a healthy one:
i. Sick fish is less timid.
ii. When taken out of water a sick fish remains calm and sluggish.
iii. When a sick fish is held in hand it lets its part of the body hang down vertically with fin folded.
iv. When a sick fish is turned to one side, its eyeball also turns following the turning of the body (a healthy fish keeps its eyeball in fixed original position).
(3) Assessment of probable cause of sickness:
This is done in two ways:
i. Evaluation of the quality of water in which the ailing fish is present. The features of the water may be assessed by analysing such features like smell of the water, transparency, colour, oxygen content, temperature, pollutant, etc.
ii. Examining closely sick fishes — diagnosis of the disease and its causative factor.
(4) Disorders by abiotic factors:
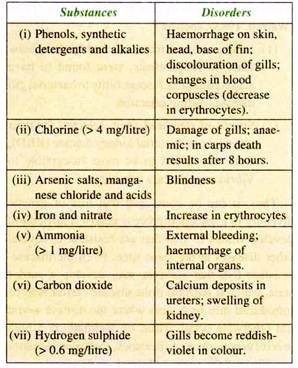
Various ingredients present in the domestic and industrial effluents may find their way into fishery resources and cause various disorders in fish.
Some of these are listed below along with the disorders caused:
(5) Major symptoms and diagnosis of common disease and parasite infection:
For examination of affected organs certain laboratory procedures are undertaken:
i. Collection of blood sample of diseased fish for haematological examination,
ii. Removal of fluid contents for examination, from boils, knots, vesicles, abscesses, etc.
iii. Culture of exudates from affected internal organs.
iv. Preparation of slide-smears from cultures grown from their exudates or directly from the diseased organs.
Diseases Encountered by Aquaculture Species:
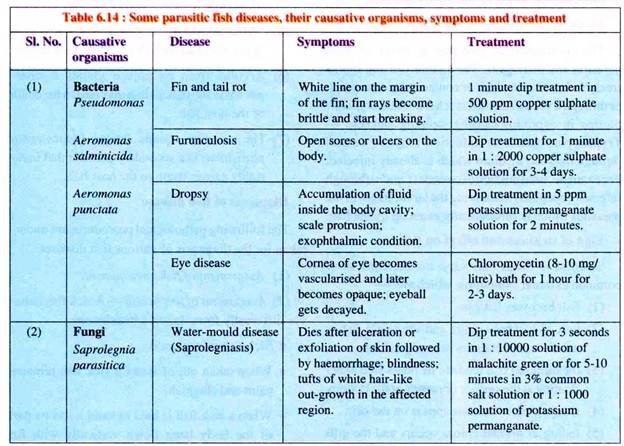
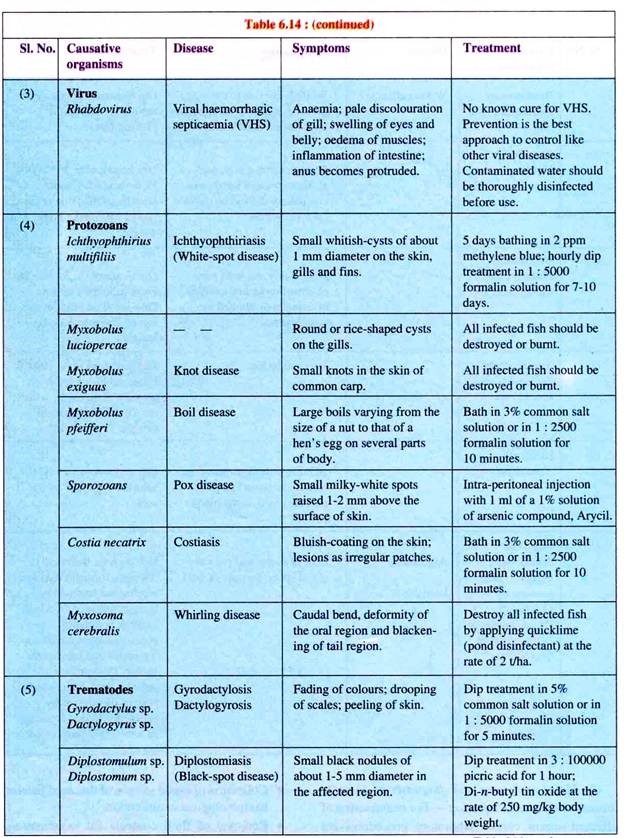
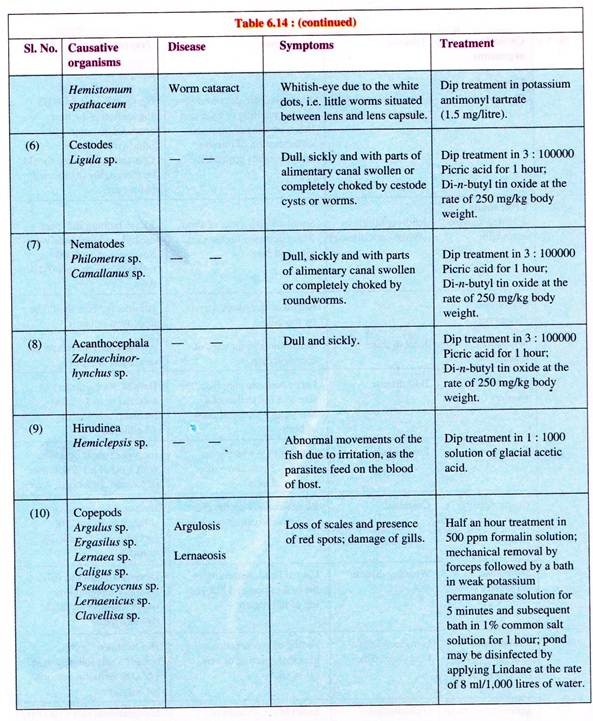
Several diseases are encountered by an aquaculturist particularly in semi-intensive and intensive systems of culture. Some of the major diseases, causative organisms, symptoms and treatment are listed in Table 6.14.
A. Nutritional diseases:
Nutritional diseases can be said to be of three types:
(a) Those arising from malnutrition.
(b) Those arising from imbalance in the major components of food.
(c) Those arising from toxic effect of the diet.
Standard artificial diets tend to eliminate these diseases. Sub-standard diets used in supplementary feeding increases the possibility of their occurrence.
(1) ‘Pin heads’ with enlarged head and slender body occur in nurseries due to starved larvae. Starvation occurs generally from inadequate supply of food or from behavioural starvation (it occurs due to overstocking).
(2) Lipoid hepatic degeneration disease is common among farm fishes caused due to overfeeding, where trash fish or pelleted diets have developed rancidity. The symptoms are swollen liver with rounded edge, anaemia followed by gills looking pallor.
(3) Nutritional gill disease occurs from panthonic acid deficiency in the diet. The symptoms are inappetence, respiratory troubles and anaemia.
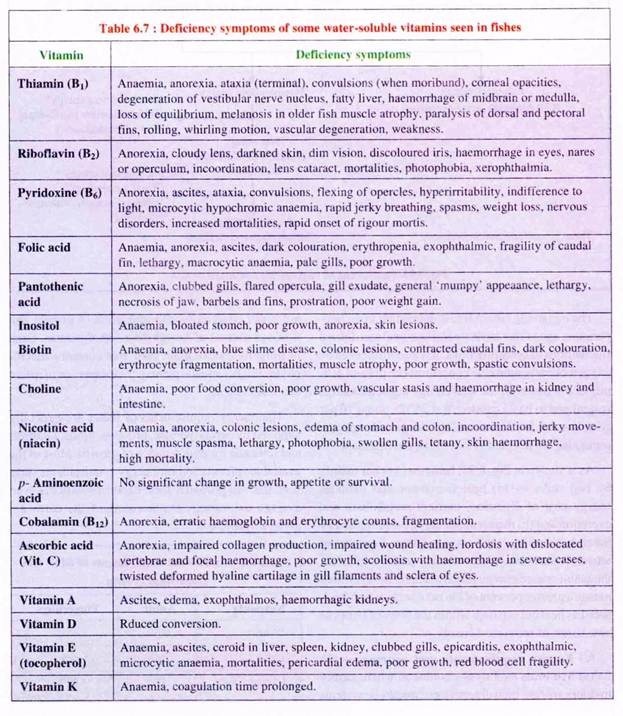
(4) Vitamin deficiency results in diseases which are enlisted in Table 6.7.
(5) Mineral deficiencies of nine essential minerals are given in Table 6.8.
B. Diseases caused by parasites and pathogens:
In aquaculture the known major diseases are caused through infection by pathogens of viral, bacterial, fungal or protozoan origin. Many of these have no known methods of therapy. Prophylaxis is the only control measure. Parasitic copepods and helminths also cause many diseases, but of lesser importance.
(a) Bacterial diseases:
Bacterial diseases have worldwide distribution. It occurs both in tropical and temperate climate aquaculture. There are several bacterial diseases of cultured fin- and shell-fish. Many bacterial infections also occur in association with viral diseases (as secondary infection). However, bacterial diseases are most feared often causing heavy loss to fish farms.
(i) Dropsy:
Abdominal dropsy of carps is caused by Aeromonas punctata. In temperate regions it occurs during spring. Symptoms include accumulation of fluid inside the body cavity, withering away of fins, scale protrusion and general edema. Intestines are inflamed and the liver and kidney are affected.
The bacterium is rod-shaped with a flagellum, measuring 1 to 1.5 mm in length. If the infected fish is in good health then the bacteria remains dormant. The weak fishes are affected which may lead to death. The disease spreads when the liquid containing bacteria escapes the body of an infected fish after post-mortem decay.
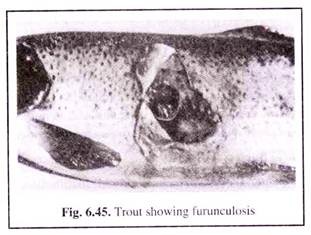
(ii) Furunculosis:
Furunculosis is caused by Aeromonas salminicida. It occurs mainly in salmonids which includes Salmo salar, S. fario, S. salvelinus, S. irridaeus, etc. The bacterium is rod-shaped measuring about 2 to 3 μm in length.
The disease has cosmopolitan distribution and also infects various species of warm-water and cold-water fishes. The name furunculosis is obtained from the blisters or furuncules that occur on the infected fishes’ skin.
The furuncules (boils) are rounded swellings appearing on the sides of the body, which contains pus—a mixture of destroyed body tissues (muscles, leucocytes, etc.) and bacteria. The swelling sometimes bursts releasing the content into the water (Fig. 6.45).
In case of massive infection, the intestine shows heavy inflammatory conditions which further spread to peritoneum and muscles. Ultimately mass mortality occurs which has been reported in trouts.
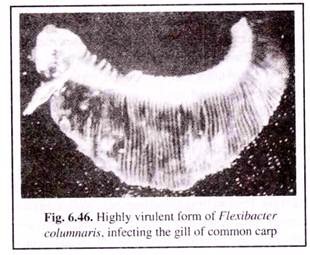
(iii) Columnaris:
Columnaris is caused by the bacterium Flexibacter columnaris. It occurs in chronic form in both cold-water and warm-water fishes worldwide. Fishes under stress due to crowding and increased temperatures are susceptible to infection.
Generally two strains have been identified — high and low virulence. The less virulent form are primarily responsible for cutaneus infection, while the high virulent ones attack the gill tissues and causes a “gill rot” condition (Fig. 6.46).
The sign of the diseases in the less virulent cases, prevalent among most species of fishes, are:
(1) Appearance of discoloured grey patches on the dorsal fin.
(2) These lesions grow and expose the underlying muscle tissues.
(3) The lesions are prominent in the mouth and head regions.
(4) The lesions may become yellow and cratered subsequently.
(iv) Gill rot:
The causative organism of gill rot, generally infecting the fingerings of grass carp, is Myxococcus piscicola. It is characterised by masses of myxo-bacteria on the gills and is associated with hyperplasia of the gill epithelium. The gill covers in case of acute infection become inflamed and are eroded by the bacteria, forming small transparent patches.
(v) Vibriosis:
The bacterium Vibrio anguillarium causes vibriosis, which occurs both in salt and fresh- waters. Vibriosis has become one of the most serious disease of cultivated marine species of fish such as rainbow trout, salmon, etc.
Vibrio generally enters the fish through surface wounds and acts mostly on the skin forming lesions. The ulcers extend deep into the muscles and internal haemorrhage, kidney damage and swollen spleen occur in acute cases.
In shrimps, infection occurs in larvae, juveniles and adult. The bacterial attack may result in septicaemia. The common sign of infection in shrimps are disoriented swimming and increasing opaqueness of abdominal muscle in juveniles and adults. Total mortality of the stock may occur.
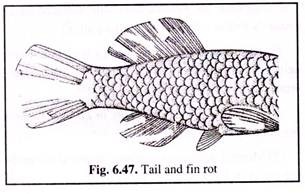
(vi) Fin and tail rot:
In fin and tail rot (Fig. 6.47), the margins of the fins become necrotic and slough away. This bacterial infection is caused by Pseudomonas. Fin and tail rots occur generally when unfavourable environmental conditions prevail. Improvement of water quality in ponds often results in cure of the disease.
(b) Fungal diseases:
Fungal diseases are among the commonest diseases of freshwater. Some forms are primary invaders of fish and other aquatic organisms.
Majority of them are saprophytic opportunists who take advantage of:
(1) Necrotic tissue associated with injuries,
(2) Bacterial or parasitic lesions,
(3) Dead and decaying eggs, and
(4) Unsanitary conditions.
Some of the important fungal diseases are:
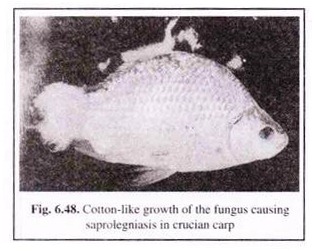
(i) Saproiegniasis (Cotton-wool disease):
Saprolegniasis is caused by Saprolegnia and some non-saprolegniaceous fungii such as Pythium and Leptomitus. This disease affects the skin and gills (in late stages) of freshwater fish and crustaceans. Small white tufts appear on skin and also on fins. They can be identified by the appearance of profusely branched, non-septate, cotton-wool-like tufts of mycelium (Fig. 6.48).
(ii) Branchiomycosis:
Branchiomycosis, the gill rot of carps, involves the attack of the fungi, Branchiomyces on the gills of carps and channel cat- fishes in summer. At the onset of the infection, the gills of the fish show deep red patches. Subsequently, necrosis of the gills sets in and the colour of the gills becomes yellowish brown. Often, secondary infection of Saprolegnia occurs at this time.
(c) Viral diseases:
(i) Infectious pancreatic necrosis (IPN):
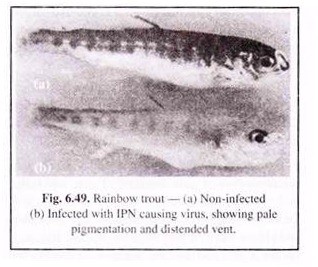
IPN is one of the important viral diseases infecting trout and salmon. This virus has also been isolated from a number of other fish species (including eels) and several species of bivalve molluscs. IPN causing virus belongs to the birnavirus group and is 70 nm in diameter. It occurs as an acute disease in fry and fingerlings. Bad husbandry, unfavourable environmental conditions and overstocking help in the outbreak.
The affected individuals show weak respiration and conclusive movements. Affected fishes show an overall pale pigmentation, exophthalmia, abdominal distention and haemorrhages on the ventral surface (Fig. 6.49). In the pyloric caecae, haemorrhages are also seen to occur. The liver and spleen become pale.
The occurance of clear or milky mucus in the stomach and anterior intestine is a characteristic feature of IPN. When the mortality rate is considerable, infected individuals swim in a rotating manner about their long axis. This whirling behaviour is a terminal sign and the fish dies within an hour or two.
Most survivors of this infection become lifelong carriers of this virus. They intermittently shed varying quantities of this virus over a long period of time, through urine, faeces, milt and eggs. Transmission of this virus from parents to the offsprings takes place through the eggs.
(ii) Viral haemorrhagic septicaemia (VHS):
Viral haemorrhagic septicaemia is economically the most important viral disease seen in cultured salmonids, especially in case of hatchery-reared rainbow trout. The disease is caused by a bullet-shaped rhabdovirus of 50 nm in diameter.
Transmission of the disease takes place by contact, by transportation or bad handling and from fish to fish through water. Rise in water temperature leads to lesser losses and the loss ceases during spring.
Symptoms of VHS include general anaemia, pale discolouration of gills, swelling of eyes and belly, oedema of muscles, inflammation of intestines, etc. On the skin sores may appear, anus becomes protruded and kidney and liver may often be affected. Bleeding occurs in gills and muscles. Signs of excitability are seen including erratic swimming.
(iii) Infectious haematopoietic necrosis (IHN):
IHN is an acute viral disease caused by a bullet- shaped virus. It occurs in the fry and fingerlings of salmons and trouts and is transmitted from fish to fish and from parent to progeny through seminal fluids or infected eggs. Mortality may occur from the sac fry stage to the yearling stage.
The symptoms are weakness, dark colouration, abdominal swelling and pale gills. The kidney gets affected. It becomes translucent and speckled with pigment cells.
(iv) Infectious dropsy of carp (IDC) and spring viremia of carp (SVC):
Infectious dropsy of carp is the most serious disease of common carp and several other fishes. IDC comprises several aetio- logically different diseases such as spring viremia of carp, carp erythrodermatitis, swim bladder inflammation, pseudomonas septicaemia and aeromonas septicaemia. The methods of treatment and prevention of these diseases are different.
SVC is caused by a rhabdovirus. It is very contagious and can cause high mortality. The symptoms include reduced movements and frequent resting, darkening of skin, protruding eyes, bleeding from eyes, fins, skin and gills, faecal casts trailing from the reddish protruding anus, accumulation of fluid in the abdomen, inflamed intestine, bleeding in the internal organs and anaemia.
The transmission of this rhabdovirus takes place from brood fish to offspring through eggs or sperm. It enters the fish through the gills.
(v) Channel catfish virus (CCV):
CC V is a serious virus disease caused by a herpesvirus measuring 100 nm. It occurs in fry and fingerlings of less than 4 months old channel catfishes in culture medium. Affected fish hangs vertically in the water and shows loss of balance (spiral swimming).
Lesions develop at the posterior part of the kidney with increased number of lymphoid cells. Lesions later develop in the liver, spleen and the digestive tract. Haemorrhages are seen in the muscles, gills, skin and fin bases.
(vi) Carp pox (CP):
In cyprinids, carp pox is caused by a virus similar to herpesvirus. The disease is characterised by proliferation of the skin. This cutaneous growth reduces marketability of the fish. However, there is seldom any mortality of the infected fish. Argulus, the carp louse acts as a virus-carrier and transmits the virus within a pond population.
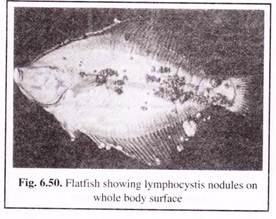
(vii) Lymphocystis:
Lymphocystis is a viral disease that occurs as whitish nodules on the fins, head and sometimes on the whole body of the fish (Fig. 6.50). It occurs in several species of freshwater, brackish water and marine fish species. In culture conditions it is highly contagious and spreads very rapidly.
(d) Protozoan diseases:
There are various protozoan parasites that live on fishes and other aquatic organisms. These parasites cause both external and internal diseases, often causing havoc in hatcheries and rearing tanks. In certain cases the infected fishes may die without showing any symptoms. Some of the important protozoan diseases are given below:
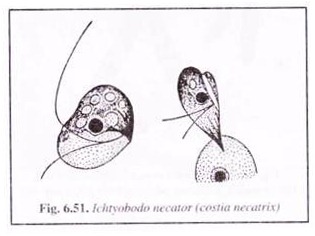
(i) Ichtyobodosis:
Ichtyobodosis is common among fishes when farmed for long periods in crowding conditions and is seen affecting various species including common carp and trout. It was previously known as costiasis. The causative agent being a flagellate Ichtyobodo necator (Costia necatrix).
The parasite (Fig. 6.51) attaches itself to the skin of the host, with its anterior end forming finger-shaped processes at the point of attachment. The parasite asexually multiples by longitudinal fission. It dies when it falls from the hosts’ body. Water acts as a medium of transmission. The parasite can tolerate temperatures varying from 2°C to 30°C.
The main symptom is the appearance of a slimy secretion on the skin, fins and the gills. Dull spots appear on the sides which subsequently becomes fused into a continuous greyish film. In advanced stages red patches may appear on the affected parts.
Fins may become folded and erosion of the tissue occurs between the rays. Infected gills become pale and get partially destroyed. If unchecked the infection may prove dangerous.
(ii) Ichthyophthiriasis (white spot itch disease):
Ichthyophthiriasis is caused by a ciliate, Ichthyophthirius multifiliis, and occurs in all species cultured in ponds including common carp, trout, etc. It causes the white spot itch disease which is fairly widespread.
Ichthyophthirius multifiliis measures 0.5-1.0 mm long. It has a round or ovoid body, with longitudinal rows of cilia on the body surface. Since the parasite can withstand a high range of temperature (25°C to 26°C), outbreak of the disease usually occur in spring and summer when the water temperature is high.
The symptoms are appearance of numerous small white specks (early infection) or white spots (advanced infection) over the entire body. The spots are due to a small chamber in which the parasites are accumulated. Mature parasites leave the fish periodically and are responsible for the spread of the disease. Epidemics break resulting in mass mortality when the fish is weakened and its resistance is low.
(iii) Knot and boil disease:
Various species of Myxobolus cause ulceration which appears either like a boil or knot or rice shaped. It occurs in the skin of common carp (Cyprinus carpio), in the gills and other body parts of tench (Tinea) and barbels (Barbus).
(iv) Whirling disease:
The protozoan, Myxo- soma cerebralis causes whirling disease which is prevalent among salmonids. Here the common sign of the disease is tail-chaising behaviour which is done rapidly. This takes place when the fish is frightened or trying to feed.
The parasite feeds on the cartilage of young host fish. In advanced stages of infection, skeletal deformation takes place including deformed skulls and gill covers and spinal curvature. In early stages of the disease, trout may develop ‘black tail’.
The infection spreads through infected fish, contaminated water and mud. The spores of Myxosoma can survive for 10-15 years in contaminated mud.
(e) Copepod infection:
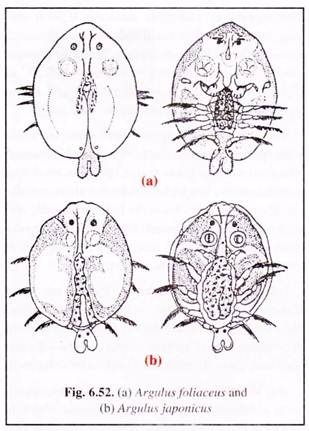
Copepod parsites are ectoparasites of the families Arguilidae, Ergasilidae and Lernaeidae. They generally infect several cultiviable fish species. Argulus foliaceus and Lemaea cyprinacea are the ones best known.
(i) Fish lice:
Infection by fish lice is one of the commonests. Argulosis is caused by Argulus species, such as A. foliaceus (Fig. 6.52a), A. coregoni, A. japonics (Fig. 6.52b) and A. giordanii. Outbreak of argulosis is often fatal causing mass mortality.
The parasite is large, the size of adult lice ranges between 4-5 mm for males and 6-7 mm for females. They are oval with flattened greyish-green body. The parasite has large suckers and a proboscis for clinging and sucking blood from the host body surface.
Carps, trouts, tench, pike, bream, eel, etc. are known to be the common victims. The affected fish shows red blotches on the skin. The fish victim makes all effort to dislodge the parasite by way of frequent scratching. Signs of nervousness are apparent in the behaviour of the fish.
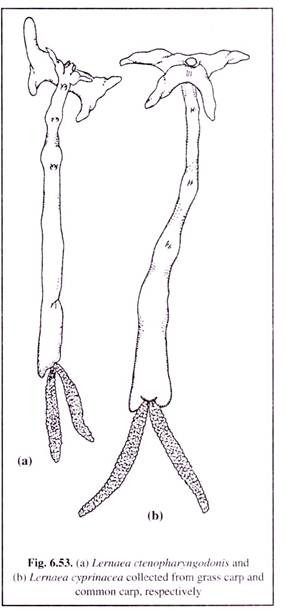
(ii) Anchor worm disease:
Anchor worm disease, better known as Lernaeosis, is caused by the species of Lernaea, such as L. cyprinacea (Fig. 6.53b) and L. ctenopharyngodonis(Fig.6.53a). This infection has been observed i n a number of freshwater fish. The mature females of these anchor worms have a long unsegmented body.
The head has branched processes, by the help of which the parasite penetrates the body of the host (Fig. 6.53). This worm has no intermediate host. The larva which is free-living, parasitises on any species of fish. However, the worm is very sensitive to salinity and can survive only on low salt concentration.
(f) Worm infections:
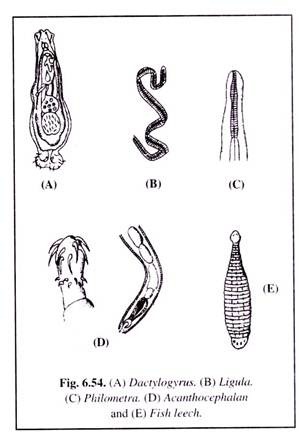
Four groups of worms are parasitic on fishes. They are flatworms (trematodes), tapeworms (cestodes), roundworms (nematodes), thorny-headed worms (acanthocephalaus) and fish leech (Fig. 6.54).
(i) Flatworm infection:
Such infection is generally caused by flatworms (trematodes) Distomum and Dactylogyrus (Fig. 6.54A), who attack the gills and cause swelling and marginal discolouration. The life cycle of these parasites are completed with the help of two intermediate molluscan hosts.
(ii) Tapeworm infection:
Such infection is caused by the tapeworm (cestode), Ligula intestinal is (Fig. 6.54B), which attains a length of 15 to 40 cm. Its infection is commonly witnessed more in wild fishes than in cultivated fishes. Fishes infected by this parasite show a swelling of the belly.
The gonads get adversely affected with loss of fertility. Death may also occur. The parasites harbour in the fish-eating water-birds where they attain their adulthood.
(iii) Fish leech infection:
Fish leech infection is caused by leeches (Fig. 6.54E) of families Rhyncobdellidae and Gnathobdellidae. Small fishes are more affected. The fishes appear restless and make vain efforts to get rid of the parasites.
C. Control of fish diseases:
To cure diseased fishes on large scale is not only difficult but also too expensive to be of any practical use. For curing diseased fishes it is fruitful only at the initial stages of the outbreak of the disease and particularly when the number of such fishes is small and the damage done is less.
However, to keep infections at a lower scale it is essential to take certain preventive measures, such as maintenance of hygiene and some prophylactic measures.
(a) Hygiene maintenance:
The preventive measures to be taken are:
(i) Periodical drying of ponds and then refilling it with fresh, good quality water.
(ii) Siltation should be avoided as far as possible.
(iii) Growth of weeds to be controlled.
(iv) Pollution of the water body is to be avoided.
(v) Sustainable stocking density is to be maintained and over feeding is to be avoided.
(vi) Water bodies should not be stocked with unhealthy or poor quality eggs and fish larvae.
(vii) Frequent handling, or transport of fishes is to be avoided.
(viii) On the first detection of the disease the affected fishes (or the dead ones) should be removed and treated separately. The dead fishes is to be buried with quicklime.
(b) Prophylaxis:
The following safety measures are to be taken:
(i) Routine disinfection of nets and other related tools are to be undertaken.
(ii) When ponds are periodically dried, they should be disinfected, each time with quicklime before water is filled.
(iii) Ponds when threatened with disease outbreak should be disinfected with either potassium permanganate (1 gm per 200 litres) or with 600 ppm benzalkonium chloride or calcium cyanamide.
(iv) Fishes before being stocked, should be given a salt bath which will not only rid them off any external parasite (Ex. Costia) but will also make them less vulnerable to future attacks.
(c) Prevention of nutritional diseases:
Nutritional diseases can be kept under control if the following measures are taken:
(i) Food of good quality, rich in vitamins but containing less of fat should be used for feeding fishes.
(ii) The supplementary feed should be given according to the recommended dose and in no case should exceed 2-5% of the total weight of fish in the pond.
(iii) The supplementary food should be distributed in a controlled manner and overfeeding should be avoided.
(d) Treatment of individual affected fish:
In the case when the disease has been identified, the affected fish is treated with medicines and quarantine baths using common salt, copper sulphate, potassium permanganate, malachite green, quicklime, formalin, lysol, etc.
(i) Bacterial diseases:
In case of bacterial infections, antibiotics, such as chloramphenicol may either be mixed with food or injected into the body at the rate of 1 to 1.5 mg per 100 gm body weight.
(ii) Fungal diseases:
The fungal infected fish is given a bath of potassium permanganate (1 gm in 100 litres) for 1 to 1½ hours, or common salt (25 gm in 1 litre) for 10 minutes, or malachite green (1 gm in half a litre and then diluted to 1 ml per litre) for 1 hour, or copper sulphate (5 gm in 10 litres) until the fish gets fatigued.
(iii) Protozoan diseases:
Fishes infected with costiasis parasite are to be given a bath of formalin (4 ml in 10 litres) for 10 minutes, while for ichthyophthiriasis, the pond water may be treated with 1 gm of malachite green per 12 square metres area thrice, at an interval of two days.
(iv) Leech parasites:
Fishes affected with leech parasite are treated with a lysol (cresol and soap in 1: 1 ratio) bath (1 ml in 5 litres) for 15 seconds and then rinsed with fresh clear water.
(v) Flatworm parasite:
The fishes attacked by Dactylogyrus should be given a formalin bath (1 ml in 1 litre) for 15 minutes, or a salt bath (25 gm in 1 litre) for 10 minutes.
(vi) Copepod infection:
In case of attack by Argulus, the fishes should be given a bath of lysol (quantity as mentioned above) or potassium permanganate (1 gm in 1 litre) for 40 seconds.
(e) Therapeutical treatment:
Therapeutic measures for treatment of fish stocks are done through two methods —
(i) treatment by immersion and
(ii) treatment via food.
In therapy of fish stocks, it is important to keep down the cost.
(i) Treatment by immersion:
This is suitable in a general way for both large and small fish populations. The immersion method requires high labour cost which far exceeds the cost of the chemicals. However, cheap medications are available, such as formalin and malachite green.
In immersion treatment, the usual practices include bath treatment for single ponds, flush treatment for raceways and flowing treatment for interconnected batteries of ponds.
(ii) Treatment via food:
Treatment measures via food is suitable only for such populations who are fed with artificial feed. In this treatment, the cost of the drug (such as antibiotics and chemotherapeutics) far outweighs labour cost. However, cheap medications, such as sulphonamides, are also available which may reduce the cost of treatment.
The drugs used are:
(1) Furazolidone is a nitrofuran, that is popular in veterinary therapy. The drug is incorporated in fish food in the case of bacterial infection. The dose recommended is 11 gm per 100 kg body weight per day for about a week.
(2) Sulphadiazine is a sulphonamide, found equally effective in case of bacterial diseases. It is administered through the diet in the ratio of 5: 1. The dose recommended is 5.5 gm per 100 kg body weight per day for about a week.
(3) Oxytetracycline is commonly used for bacterial therapy of vibrosis and ulcer disease in fishes. The recommended dose is 7.5 gm per 100 kg body weight per day for 1 to 2 weeks.
(4) Di-n-butyl tin oxide is effective against most parasites of the fish gut other than protozoans. The dose generally applied is 25 gm per 100 kg body weight per day for 3 days.
(5) Epsom salt or magnesium sulphate is found useful for the treatment of protozoan parasites and is mixed with the diet.
Epizootic Ulcerative Syndrome (EUS):
The outbreak of Epizootic Ulcerative Syndrome took place for the first time in India from Tripura state in 1988. It caused heavy loss to fishes in the wild as well as in farms, practically engulfing all freshwater bodies. It attacked a large number of species such as Puntius, Clarias, Heteropneustes, Mystus, Mastacembelus, all carps, etc.
The clinical symptoms and pathological features were:
(1) Ulceration of the skin.
(2) Erosion of epidermis and dermis with deep large ulcers.
(3) Hypodermis and muscles showed marked inflammatory haemorrhage.
(4) Raised areas of emudation and erythema were of common occurrence.
(5) Necrosis of vital organs like kidney and liver.
(6) The virulence was noticed in 3 stages:
(i) Initial stage marked with general haemorrhagic ulceration of the skin and scale pockets.
(ii) The middle stage showed heavy loss of scales from the epidermis.
(iii) The final stage witnessed haemorrhage and necrotic ulcers.
The causative pathogen includes all major pathogenic forms — bacteria in the skin, fungi in the liver, protozoans in the kidney and Argulus and Gyrodactylus on the gills. Viral infection was also found to be associated. All these pathogens were observed to be due to secondary infection. The primary pathogen is yet to be detected.
The control measures of this outbreak include application of lime (500 kg per hactare), potassium permanganate at the rate of 2-4 ppm and a chemical compound that is marketed as ‘CIFAX’.