The following points highlight the top five techniques adopted for induced breeding. The techniques are: 1. Fish Pituitary Gland—Collection and Preservation 2. Preparation and Preservation of Pituitary Extract 3. Collection, Care, Identification and Selection of Breeders 4. Assessment of Pituitary Extracts 5. Collection of Eggs and Transfer to Hatcheries.
Induced Breeding: Technique # 1.
Fish Pituitary Gland—Collection and Preservation:
(a) Fish Pituitary Gland:
Pituitary gland of a fish is small, soft bodied and whitish in colour. In carps it is more or less round in shape. It lies on the ventral side of the brain behind the optic chiasma.
ADVERTISEMENTS:
It is placed in a concavity on the floor of the brain box, called sella turcica, and is covered by a thin membrane, called duramater. In some fishes the pituitary is attached to the brain by a thin stalk, the infundibular stalk and is called Leptobasic. In other fishes the gland is without stalk and is called Platybasic.
(b) Donor Fish:
Donar fishes are those from whom pituitary glands are collected. Pituitary glands should be collected from fully ripe gravid fishes, as the gland is most potent at the time of breeding or just before spawning. Recently killed or live fishes are supposed to be the best donors for the collection of pituitary glands. However, glands can also be collected from donor fishes which are well preserved in ice or kept in cold storage.
Certain points should be noted at the time of pituitary gland collection:
ADVERTISEMENTS:
(1) The potency of the gland decreases after spawning. So glands should not be collected from i immediately spawned fishes. However, glands from induced- bred carps immediately after spawning are found to be fully potent and can be used for further hypophysation work.
(2) Glands collected from immature or spent up fishes usually do not give satisfactory results.
(3) Glands should not be collected from rotten or semi-rotten fishes.
(4) At the time of collection, care should be taken not to damage the gland as the torn gland loses its potency.
ADVERTISEMENTS:
(5) Another big problem is that pituitary glands are generally collected from fishes of variable maturity and hence the potency of the extracts shows considerable batch variation.
(6) There is no qualitative difference in the pituitary gland of male and female donor fishes.
(c) Collection of Pituitary Gland:
Pituitary gland is located in the floor of the brain box and can be collected by one of the following ways:
(i) By Cutting and Removing a Portion of the Brain-Case:
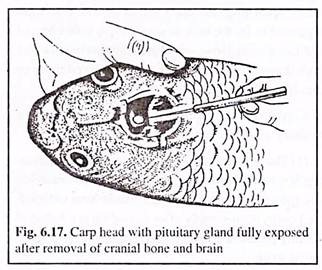
The skull or brain-case above the brain is cut with the help of a sharp butcher’s knife. The cut scalp is then removed. The grey matter and fatty substances over the brain are removed. Then, with the help of a tweezer, the olfactory and optic nerves at the, interior part of the brain are detached.
The entire brain is then lifted up and laid back. The pituitary gland (Fig. 6.17) is then located at the floor of the brain, ventral to the optic chiasma. The gland is then carefully picked up (without damaging it) by a pair of fine tweezer and kept in a dark bottle containing absolute alcohol.
Collection of the gland by this method results in damaging of the fish head which would then be a problem of being sold and thus, the cost of collection of a single gland would be very high.
ADVERTISEMENTS:
(ii) Collection of Gland through the Foramen Magnum:
By this method pituitary gland can be collected from the cut fish head. In the fish market where cut fish heads are available, the foramen magnum is first exposed by removing vertebral parts adhering to the skull by the help of a sharp knife. The brain lies on the ventral side of the foramen.
The grey matter and fatty substances over the brain is then removed by the help of blunt end of the forcep. Care is taken so that the brain is not detached or damaged. With the help of the forceps, the anterior end of the brain is then detached. The brain is carefully lifted out of the foramen magnum. The pituitary gland is then located and picked up carefully by the help of tweezers.
This method is easier and less time-consuming. It is also more economical as the cut head can be sold after the removal of the gland, as much damage has not been incurred.
(d) Preservation of Pituitary Gland:
For induced spawning, pituitary glands can be used in the fresh state, but it is generally preserved for future use. The gland may be immediately frozen and stored in frozen condition in a refrigerator.
Otherwise, it can be preserved by one of the following ways:
(i) Preservation in Absolute Alcohol:
Immediately after collection the glands are put in absolute alcohol in a dark coloured bottle. After 24 hours, the glands are washed with absolute alcohol and kept in a phial with fresh absolute alcohol. Pure absolute alcohol must be used because the hormones are soluble in water.
Absolute alcohol dehydrates and defattens the glands. Occasional changing of the alcohol helps in proper dehydration and defattening of the glands and keep them in good condition for a long period. The phial is made air-tight and kept in a cool, shady place at room temperature. It may be kept inside a dessicator containing anhydrous calcium chloride.
Preservation of pituitary gland in absolute alcohol is preferred in India as very few fish-culturists can afford to have a refrigerator. Moreover, alcohol- preserved glands have given positive results than acetone-dried glands when used on Indian carps.
(ii) Preservation in Acetone:
The ‘Acetone- Dried Pituitary’ is largely in use in Russia and U.S.A. where refrigeration facilities are common. It is available from a number of reliable suppliers in the U.S.A, Israel and Germany. Immediately after collection of the pituitary gland, it is put in acetone containing, phials.
The phials are then kept in a refrigerator or placed on dry ice. The acetone should be changed several times over a 24-hour period to ensure that the glands have been properly dehydrated and defattened. After 36 hours of storage in the refrigerator, the phials are taken out and kept at room temperature. After some time the glands are taken out of acetone and dried on a filter paper at room temperature.
It is then stored either intact or after being grounded to a powder, in sealed air tight sterile phials, in a refrigerator or preferably in a -20°C freezer. Glands stored under these conditions retain their potency even after 15 years.
Induced Breeding: Technique # 2.
Preparation and Preservation of Pituitary Extract:
The pituitary extract is then prepared from the gland for injecting it into the recipient fish (breeders). The extract can conveniently be prepared well in advance and used when needed, thus saving time and trouble of preparing it before every induced breeding operation.
(a) Preparation of Pituitary Extract for Injection:
The quantity of gland required for injection is first calculated from the weight of the breeders to be injected. The required quantity of the glands are then taken out of the preserved phials and kept on a piece of filter paper (1-2 minutes) for drying or evaporation of the alcohol. On the other hand, acetone-dried glands are straight away taken from the phials for maceration.
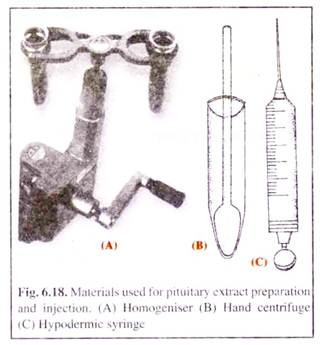
Appropriate amount of the entire or dry powdered glands are weighed. The segregation and mixing of many glands of variable gonadotropic potency has the advantage of producing a single extract of consistent potency. The glands are then taken into a tissue homogeniser with a little quantity of distilled water or 0.3% saline solution (for I.M.C.) and thoroughly homogenised.
Further amount of the same solvent is then added to achieve a concentration of 20-50 μg of dried pituitary material per ml of the solvent. The amount of the pituitary gland per ml of the media may be increased or decreased according to the requirement of the suspension to be injected. It is suggested that a maximum of 1.0 ml and a minimum of 0.1 ml suspension should be injected to a breeder at a time.
The macerated gland suspension is then poured into a centrifuge tube (Fig. 6.18) and centrifugation is done at 3000 revolutions per minute (rpm). The suspended particles of the crushed tissue and other solid particles settle down at the bottom of the centrifuged tube and the clear solution containing the hormones is then decanted into a small beaker to be taken into a hypodermic syringe for injection.
(b) Preservation of Pituitary Extract:
Preservation of pituitary extract can be done in glycerine. In this method, a known quantity of pituitary glands, after homoginising in distilled water, is diluted to 1/3 of the total volume of the extract intended to be made with water. The suspension is kept in refrigerator for 24 hours. Pure glycerine is then added to the extract to make up the intended volume.
For example, 3 ml of the extract will have 1 ml of distilled water and 2 ml of glycerine. The extract with glycerine is again kept in the refrigerator for another 24 hours, after which it is centrifuged. The supernatant fluid is then transferred into ampoules and sealed for future use. The ampoules should be properly labelled with the details of hormone concentration and date of preparation.
Glycerine preserved extracts must be prepared one or two months prior to the onset of the breeding season. It is found to retain its potency for about 1 year or more if properly refrigerated.
Induced Breeding: Technique # 3.
Collection, Care, Identification and Selection of Breeders:
(a) Collection and Care of Breeders:
The primary requirement for fish breeding work is collection and stocking of healthy recipient fishes or breeders. For successful breeding, selection of healthy breeders is essential. Generally 1.5 to 5 kg weight of Indian Major Carps are preferred for economical breeding.
The breeders may be collected from any water resources, such as reservoir beel, jheel, canal, tanks, fish-farms, etc. They are stocked at the rate of 500-1000 kg of fish per acre or 1250-2500 kg per hectare water area. If the breeders are collected during winter season, they should be stocked in a large tank and prior to the onset of breeding they should be sex-wise segregated.
If they are collected a few days or a month prior to the commencement of breeding, then they are stocked sex-wise in two separate tanks, called segregation tanks. Segregation of the sexes prior to injection (although not essential) increases the affinity of maturing during the breeding procedure and gives encouraging results.
For maintaining healthy growth, the breeders are fed with artificial feed, such as powdered cottonseed, rice bran, oilcakes (ground-nut, mustard, etc.) or a mixture of oil cakes and rice bran. They are generally fed daily for 15-30 days before injection at the rate of 10% of their body weight.
During netting, proper care should be taken and rough handling should be avoided as it may cause injury to the breeders. Periodic examination of the breeders is done to see the condition of the gonads and parasitic infections, if any. In case of any infection, the breeders are dipped for half an hour i n a solution of 1 ppm potassium permanganate or acriflavine and then released back into the pond.
(b) Identification of Male and Female Breeders:
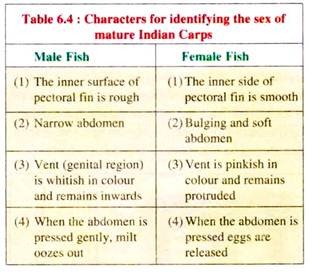
Although fishes are sexually dimorphic, it is difficult to differentiate their sexes morphologically in most fishes. In carps, the secondary sexual characters are not very prominent and conspicuous by which the sexes can be distinctly identified.
However, some morphological characters develop during the breeding season in both male and female IMC which serve as an identification key. These characters listed in Table 6.4 are more or less the same in all the species of various genera of carps.
(c) Selection of Breeders:
Success in induced breeding largely depends upon proper selection of suitable male and female breeders. Selection of male brood fish is easy, as a fully ripe male oozes milt freely on being gently pressed at the belly. On the other hand, selection of a suitable female is a major difficulty with hypophysation.
The identification characters such as tubercles or roughening of the surface of the body, changes in colouration, softening and rounding of the abdomen and reddening and protrusion of the anal papilla and vent have all been used, but each is notoriously unreliable. The most reliable character is taking the egg and examining it under the microscope.
Pituitary extract injections should only be given to those female fishes in which the nucleus is on the periphery of the egg shell. Less mature females should be returned to the holding ponds or stocking tanks for re-examination at a later date. However, such practices in India is not possible and the farmers have to relay on the secondary sexual characters (Table 6.4).
Induced Breeding: Technique # 4.
Assessment of Pituitary Extract:
(a) Dosage of Pituitary Extract:
For successful induced breeding, it is essential that proper dosages of pituitary gland is assessed. Standardisation of dosage is dependent upon a number of factors of which the size and the sexual stage of maturity of the breeders, particularly the females, are of utmost importance.
The dose of the pituitary injection to be given is calculated in relation to the weight of the breeders to be injected. It has been found that administration of a preliminary low dose in the female breeders followed by a higher effective dose after 6 hours has proved more successful than a single knock-out dose.
On the other-hand, the male spawners are far more tolerant of the farm environment than that of female fish. For this reason male fishes require much lower doses of pituitary material for the induction of spawning by hypophysation.
The female breeders of IMC are given a preliminary dose at the rate of 2-3 mg of pituitary gland per kg body weight. After 6 hours, the females are given a second higher dose of 5-8 mg per kg body weight. During this time the males receive their only dose, at the rate of 2-3 mg per kg body weight.
A third dose of injection is generally not recommended. However, if the condition of the female breeder is found suitable and also the weather condition, then a third injection may be given. It is given 12 hours after the second injection at the rate of 4-7 mg per kg body weight to the females. In case, it is decided not to give the third injection, then the breeders are released back into the stocking pond.
The pituitary extract dose given to the exotic carps is slightly higher than that given to IMC. The females of the exotic carps are usually given an initial dose of 3-4 mg per kg body weight, followed by the second dose of 7-10 mg per kg body weight at an interval of 6 hours. At this time the males are given the only dose of 3-6 mg per kg body weight.
(b) Injection Methods:
Different methods of injection are in practice, like
(i) Intra-muscular,
(ii) lntra-peritoneal and
(iii) Intra-cranial. However, intra-muscular injections are more commonly practised by fish-breeding workers.
In India both intra-muscular and intra-peritoneal injections have been tried but the former method has been found to be more effective and easier to follow. Intra-muscular injections are usually administered in the region of the caudal peduncle or near the shoulder region.
Care must be taken to avoid the lateral line area. In case more than one injection is to be administered, it is better to give the second injection on the other side (i.e. the side which did not receive the first injection).
The most convenient hypodermic syringe used for injection purpose is a 2 ml one having 0.1 ml graduations, preferably with a locking arrangement for convenient use. The size (thickness and length) of the needle for the syringe depends upon the size of the breeders to be injected. Generally B.D.H. needle No.22 is used for 1-3 kg carp breeders, needle No. 19 for larger (above 3 kg) and No. 24 for smaller (below 1 kg) ones.
(c) Injection Technique:
For giving injection to the breeders, the requisite quantity of gland suspension is taken in a hypodermic syringe. The brood fishes are then taken out of the hapa, where they were sex-wise segregated, and weighed. They are then brought one by one for injection and placed on a small field table provided with a cushion. The cushion is made up of wet pieces of nets, hapas, cloth, etc.
Two persons are required at the time of injection; one of them holds the head of the fish pressed gently against the cushion while the second presses the tail with one hand and with the other gives the injection on the caudal peduncle area (Fig. 6.19).
For intra-muscular injection, the needle is first inserted under a scale parallel to the body of the fish and then pierced into the muscle at an angle of 45°, so that no damage is done to any of the scales.
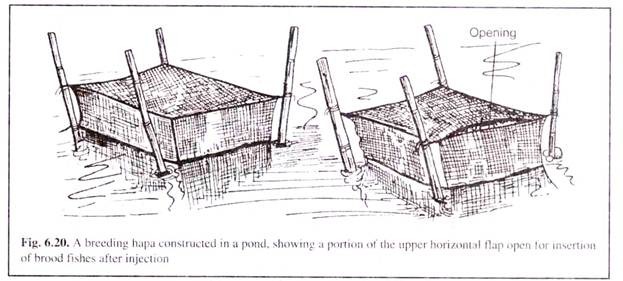
The entire operation is done close to the water body where they would be introduced after the final injection. The breeders, after the final injection are immediately released into the breeding hapa (Fig. 6.20) or Bangla Bundh (Fig. 6.15) or breeding pool (Fig. 6.21).
(d) Injection Time:
Injection may be given at any time of the day or night, as spawning can occur at any time. But since a low temperature is helpful for spawning, injection may be given on a cool day or in the evening or night when the temperature is fairly low.
(e) Spawning:
In case where a single high dose is administered, spawning generally takes place about 6-9 hours after the injection. In case of two injections (preliminary and a second dose to the female, at an interval of six hours and only a single dose to the males) spawning occurs within 3-6 hours after the second injection.
A few hours after the final injection sex play between the female and male is noticed. This is best observed in the Bangla Bundh as described earlier. Spawning normally is influenced by low temperature (25-30°C), rain water, slight drizzling and cool weather. The average fecundity rate of IMC has been estimated to be about 2 lakh eggs per kg body weight.
Control of Reproduction/Spawning:
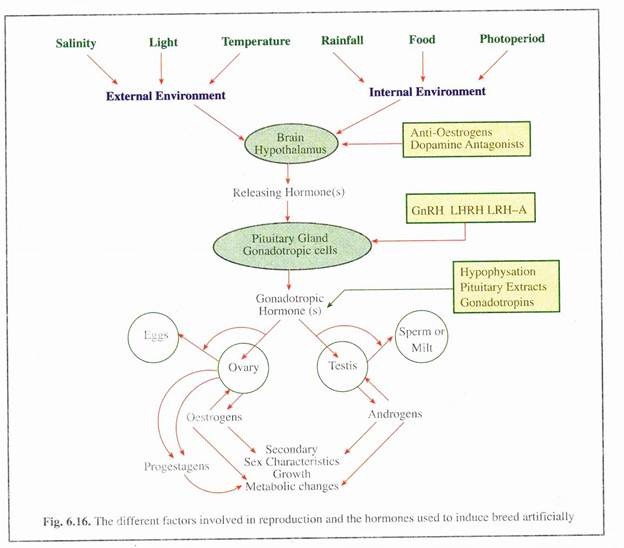
Many hormones are involved in the control of maturation and spawning but those produced by the hypothalamus, pituitary and the gonad itself are of primary importance. A correct combination of environmental factors is required for maturation, ovulation and spawning. It brings about an accelerated release of gonadotropin from the pituitary into the bloodstream.
The major-events in the control of reproduction and some of the steps which are accountable to artificial manipulation are given in Fig. 6.16. If the reproductive cycle is to be modified for aquaculture advantage, then an understanding of the relationship between the various components involved in the control of gonadal function is essential.
The Control of Reproduction Comprises of the Following Steps:
(1) The various internal and external environmental factors act upon the hypothalamus.
(2) In response to the changing conditions of these factors the hypothalamus secretes small peptide hormones, called releasing hormones.
(3) These peptides pass a short distance to the ventral surface of the brain, where they control the activity of specific gonadotropic cells in the pituitary gland.
(4) These cells then secrete gonadotropic hormones which are carried by the blood to the gonad of the fish.
(5) Here, these cells control all the structural and functional changes in the testes and ovary.
(6) One important influence of gonadotropic hormones is the induction of hormone secretions from the gonads, which either act independently and/or in concert with the gonadotropic hormones which directly control all the aspect of gonadal growth and maturation.
(7) In Female Fishes Two Main Ovarian Hormones are Produced:
(i) Oestrogens, which include oestradiol – 17β and oestrone, and
(ii) progestagens, 17α- 20β-dihydroprogesterone and 17α-hydroxyprogesteron.
(8) In males, the major testicular hormones produced, referred to as androgens, are testosterone and 11-ketotestosterone.
(9) All of the above gonadal hormones have a steroid structure which is same or similar to that of the corresponding hormones of higher vertebrates.
Fishes of commercial importance vary in their reproduction strategies and tactics. However, despite their diversity, all of them have their reproductive cycles controlled by the same cascade of endocrine controls. By hormonal administration and environmental manipulation, fish culturists have been able to alter the times of spawning of a wide range of fish species for commercial advantage.
Factors Conducive for Successful Spawning:
(1) Availability of fully matured and ripe male and female breeders.
(2) Collection of pituitary glands from fully gravid fishes.
(3) Proper assessment of dosage of pituitary hormone.
(4) Conducive physico-chemical factors of environment for spawning are:
(i) Optimum dissolved oxygen ranges generally between 6-7 ppm.
(ii) Optimum pH ranging between 6.0-8.0.
(iii) Lunar periodicity.
(iv) Optimum temperature ranging between 28-30°C.
(v) Lightning and thunder shower.
(vi) Stimulation effect of silt particle pressure on the body of the spawners. This pressure can be measured by conductivity meter.
(vii) Electro-magnetic properties of water within a limited range.
(viii) High turbidity (2000-2500 ppm).
(ix) Stimulation on mature fish by minerals in solution or suspension in water.
(x) Current of water.
The resultant effect of the above stated physiochemical factors provide stimulation for sex play and spawning. Viewed from the point of physiology of the spawners, these external stimulii help in releasing necessary hormones first from the pituitary gland, for stimulating gonads as well as for the final release of ova and sperm for fertilisation.
(f) Fertilised Eggs:
As the female liberates the eggs in a stream, the male oozes its milt and the eggs thus gets fertilised. After fertilisation the eggs swell up considerably within 10-15 minutes, owing to the absorption of water through the vitelline membrane.
The fertilised eggs become crystaline transparent and non-adhesive with a reddish tinge in catla, pale reddish in rohu, light pale brownish in mrigal and pale bluish in calbasu. The sizes of the fully swollen eggs of the four IMC’s vary within 2.5 mm to 6.5 mm in diameter.
The unfertilised eggs also swell up in water but its colour becomes opaque whitish and their size is much smaller than the fertilised eggs.
(g) Stripping or Artificial Insemination:
In case of Chinse carps artificial insemination is done through stripping. In stripping method the ‘eggs’ and ‘milt’ are forcibly extruded out by applying pressure on the belly of breeders when they are ready to breed.
Stripping or artificial insemination is done owing to probable reasons given below:
(1) The eggs on some occasions, when naturally released remain unfertilised or even show some initial cleavage. After which further development stops altogether and the egg turns opaque. This may be due to a sort of parthenogenetic development of eggs. Such eggs cannot be fertilised by sperms.
(2) Due to non-synchronisation spawning behaviour of the male and female in natural spawning, the eggs may remain unfertilised.
(3) The eggs may remain unfertilised due to poor or inadequate quantity and quality of the milt.
(4) The females may not be in prime stages of maturity.
(5) Adverse environmental factors (like rise in temperature) may affect spawning.
Two methods of stripping are generally practised — dry method and wet method:
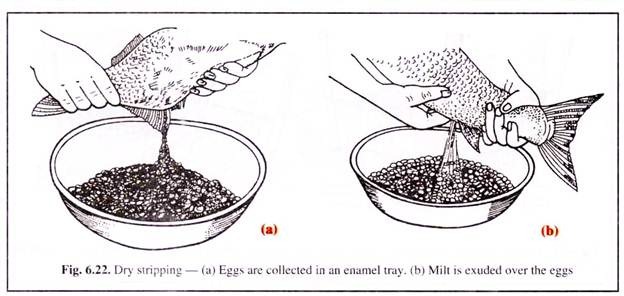
(1) Dry Stripping:
The dry method of stripping has been found to be more convenient and effective and is widely practised in India. In this method after 3-4 hours of the second infection, the female fishes are examined, whether they have become fit for stripping. The female breeders become fit for stripping when the abdomen becomes very soft and eggs oozes out freely on applying slight pressure on the abdomen.
The females are first stripped of eggs in an enamel tray (Fig. 6.22). The abdomen of the males are then pressed and the milt is then added over the eggs. The milt is then thoroughly mixed with the eggs, taking the help of a plastic spoon or bunch of feathers.
After few minutes the fertilised eggs are washed thoroughly to remove the excess milt and blood clots. The fertilised eggs are then left immersed in water for 4-6 hours for water hardening of eggs.
(2) Wet Stripping:
In some countries wet method of stripping is also practised. In wet stripping 0.3% saline solution is used. The salt solution is taken in an enamel tray and the males are made first to exude milt. Then the female breeder is taken and the eggs are stripped into the tray. Generally the sperms of Chinese carp remain active up to 2-3 minutes in 0.3% saline, while they die much earlier in ordinary water.
Induced Breeding: Technique # 5.
Collection of Eggs and Transfer to Hatcheries:
Eggs should be collected when the embryo starts the twisting movement, because by that time the eggs get properly water hardened and can withstand sufficient strain. Eggs are collected from the hapa by means of a cup or beaker and poured into a bucket with a small amount of water.
In the case of Bangla Bundh, the eggs are collected with the help of a ‘chotjal’ and transferred to a ‘hundi’. While in case of breeding pool, the eggs are directly passed on to the Chinese Hatchery through underground inter-connecting pipe system.
The breeders are then collected and the weight of the breeders after spawning is noted. The difference in weight before and after spawning gives an idea of the quantity of the eggs laid. The breeders are then transferred back into the pond.