Here is a list of top three experiments to demonstrate mitosis and meiosis in animals: 1. Squash preparation of onion root tips to observe stages of mitosis 2. To prepare slide for the study of meiosis 3. Preparation of polytene chromosomes.
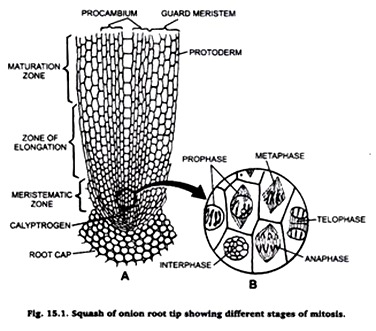
Experiment # 1. Squash preparation of onion root tips to observe stages of mitosis.
Requirements:
Onion root tips fixed in F.A.A. or Carney’s fluid, microscopic glass slide, cover-slip, acetocarmine, spirit lamp, blotting paper and microscope.
Procedure:
ADVERTISEMENTS:
Take a drop of acetocarmine on a clean microscopic slide and put on it one or two root tips. Place a cover-slip over it and tap it gently by a needle. Warm the slide over the flame of a spirit lamp and then put a blotting paper over, press it smoothly by your thumb. Examine the slide under microscope.
Result:
The cells and their chromosomes are spread out and become distinct. Carefully observe different stages of mitosis. Draw their diagrams in your practical note book.
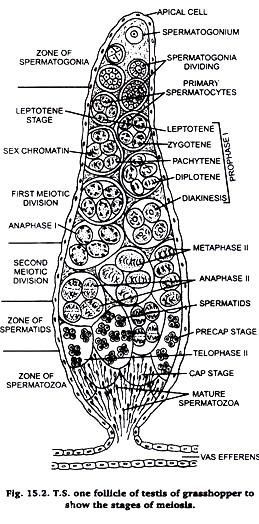
Experiment # 2. To prepare slide for the study of meiosis.
Requirements:
ADVERTISEMENTS:
Living grasshoppers, chloroform, normal saline, Carney’s fluid, acetocarmine, slides, cover-slip, blotting paper and microscope.
Procedure:
Take a chloroformed grasshopper and dissect it in normal saline. Take out its tests and fix them in Carnoy’s fluid for 2-12 hours. Take a small lobe of testis and stain it in acetocarmine. Put the stained lobe on a clean slide and cover it with a coverslip. Adopt squash preparation technique as described in case of onion root tip. Examine the prepared slide under microscope.
Result:
ADVERTISEMENTS:
The cells of testis lobes are spread our and become distinct. Carefully observe different stages of meiosis under microscope (Fig. 16.1) and draw them in your practical note book.
Note:
Meiosis is best studied in this sections of testis of grasshopper stained in Heidenhain’s iron haemotoxylin. Hence, this method is also described here.
Heidenhain’s Iron Haemotoxylin Method:
Take a lobe of Carnoy’s fixed testis and follow usual procedure of microtomy to get thin about 5 µ thickness sections. Dewax the sections and come down to water (xylol-absolute alcohol-90% alcohol-70% alcohol-50% alcohol-water). Keep the sections is 3% iron alum for 30 minutes to an hour. Wash the sections in distilled water and stain in 0.5% solution of Heidenhain’s, iron haemotoxylin.
Wash the sections in distilled water twice. Differentiate the sections in 1.5% iron alum. Wash the sections in distilled water twice and then in tap-water for at-least 30 minutes. Counterstain the sections in a saturated solution of orange G is 90% alcohol. Dehydrate the sections, clean in xylol and mount in D.P.X. or Canada balsam.
Result:
The nuclear components are stained in deep blue colour and cytoplasm in orange colour. Examine the slide carefully and note various stages of meiosis.
Experiment # 3. Preparation of polytene chromosomes.
ADVERTISEMENTS:
Polytene chromosome is one of the giant chromosomes found in animals and plants. In animals it is found in the salivary glands, Malpighian tubules, the epithelial cell lining of the gut and in the fatty cells of the larvae of certain Diptera.
The polytene chromosomes of salivary glands in Drosophila larvae can be demonstrated easily as laboratory. It can also be demonstrated in the larva of Chironomous fly.
A. Preparation of Polytene Chromosomes from Drosophila Larva:
Preparation of culture:
Take a small specimen jar. Make a mixture of the pulp of apple, banana and lemon. Take 2 gm. of moldex and add it in 40 ml boiling water. Cool it and add in pulp of fruits prepared.
Place the jar in the culture of Drosophila flies, keep it open for 2-3 days during which some of the flies will visit the jar for feeding and lay eggs. Now cover the jar with fine muslin cloth. Within a week larvae will appear. Observe carefully for 3rd instar larvae which will be white coloured.
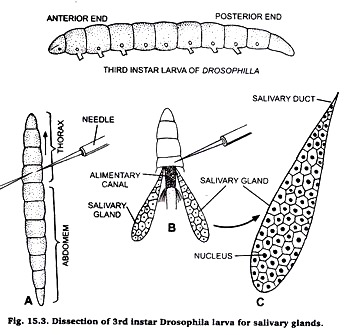
Dissection of 3rd instar larva for salivary gland:
Take a few drops of saline on a clean slide and put the 3rd instar larva in it. Locate the junction of thorax and abdomen. Take two needles, one in each hand. Press the first needle firmly on the posterior end of thorax and other needle at the junction of thorax and abdomen.
Pull the second needle so that abdomen is separated from head and thorax. Then press the thorax with a needle and observe that the salivary glands are seen floating in the saling water on the slide.
Preparation of slide:
Take a clean slide, put a drop of acetocamine on it. Transfer the salivary glands in acetocarmine on slide and cover it with a cover-slip. Leave it for 10 minutes and then warm it gently. Adopt squash technique as described in case of onion root tip. Observe the slide under microscope and for details observe under high power of microscope.
Comments:
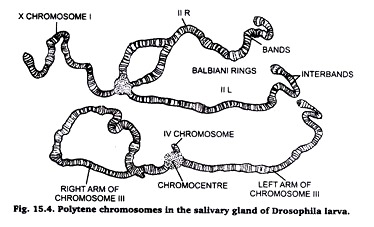
1. These are large-sized, hence, called giant chromosomes.
2. These chromosomes present alternate pattern of dark bonds and light inter-bands.
3. The dark bonds contain rich amount of DNA and RNA, and composed of much coiled chromonemal thread.
4. The light bands contain large amount of proteins and little amount of DNA and RNA.
5. A polytene chromosome is multi-stranded; it is formed of large number of chromonemal threads or strands.
6. A polytene chromosome exhibits puffs and Balbiani rings at certain points. The puffs are made of lateral extensions of bonds of chromonemalstrends into side loops.
7. The puffs and Bulbiani rings are related with the metabolic activities of the chromosomes.
8. These chromosomes help in the synthesis of proteins, nucleic acids and formation of nuclear material.
9. These were discovered by Balbiani in 1881.
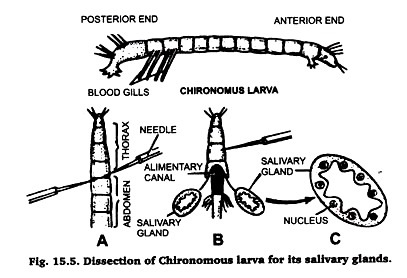
B. Preparation of Polytene Chromosomes from the Larva of Chironomous:
Chironomous larvae are found in abundance in ponds, spools, ditches and in drains. These are easily identified due to their red coloured body because they possess haemoglobin in their blood (hence also called bloodworm). Collect these larvae in ajar with a little amount of water from any of the sources listed above.
Separate the larvae from water and place them in normal saline. Identify its anterior and posterior ends by the presence of blood gills at a its posterior end. Then proceed, for taking out its salivary gland, in the same very as described in case of Drosophila larva (Fig. 15.5). Prepare the slide by following the same technique.