In this article we will discuss about the Cell Cycle and Cancer:- 1. Introduction to Cell Cycle 2. Regulation of the Cell Cycle 3. Regulators 4. Growth Factors and the D-Type Cyclins 5. Inhibitors 6. Involvement of Oncogenes and Anti-oncogenes.
Contents:
- Introduction to Cell Cycle
- Regulation of the Cell Cycle
- Regulators of Cell Cycle Progression
- Growth Factors and the D-Type Cyclins
- Inhibitors of Cell Cycle Progression
- Cell Cycle and Cancer – Involvement of Oncogenes and Anti-oncogenes
1. Introduction to Cell Cycle:
The cell cycle in most cells consists of four co-ordinated processes: cell growth, DNA replication, separation of duplicated chromosomes into two nuclei, and cell division.
ADVERTISEMENTS:
In prokaryotes, cell growth and DNA replication take place throughout the cell cycle and duplicated chromosomes are distributed to daughter cells in association with the plasma membrane. In eukaryotes, however, the cell cycle is more complex and is controlled by a conserved regulatory apparatus as well as extracellular signals.
Phases of Cell Cycle:
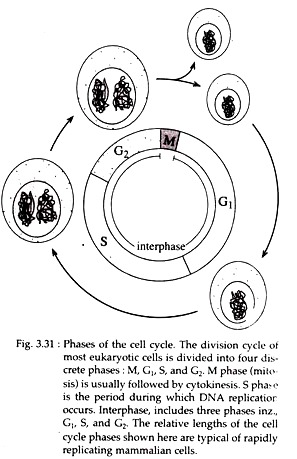
The cell cycle is typically divided into two major phases based on different cellular activities, readily visible with microscope. These are M-phase (M = mitotic) and interphase. M phase consists of two stages and interphase consists of 3 stages (Fig. 3.31).
M-phase includes:
ADVERTISEMENTS:
(i) The process of mitosis during which duplicated chromosomes are separated into two nuclei, and
(ii) Cytokinesis, during which the entire cell is physically divided into two daughter cells.
Mitosis is a dynamic period of vigorous and continual activity but in this period synthesis of macromolecules is generally shut down. The actual process of mitosis occupies only a small part of the cycle, usually about an hour.
The preparations for an upcoming mitosis occur during interphase the initial phase of the cycle. Approximately 95% of the cell cycle is spent in interphase. Cytologically (under light microscope) interphase is characterized by the absence of visible chromosomes. Instead, a distinct nucleus is observed filled with chromatin that has formed as the chromosomes have unfolded and uncoiled following the previous mitosis.
ADVERTISEMENTS:
At the molecular level, interphase is the time during which both cell growth and biochemical activities like DNA replication occur in an orderly manner in preparation for cell division. The cell grows at a steady rate throughout the interphase, with most dividing cells doubling in size between one mitosis and the next. However, DNA is synthesized only during a part of interphase i.e., S-phase.
The initiation and completion of DNA synthesis can be detected by monitoring the incorporation of radioactive DNA precursors such as 3H-Thymidine, a process called autoradiography. Investigations have revealed two other periods during interphase, before and after S-phase, when no DNA synthesis occurs. These are designated as G1 (gap 1) and G2 (gap 2) phases, respectively.
The interval between the end of the DNA synthesis and the start of mitosis is G2 phase (Fig. 3.31). G2 nuclei do possess some mechanisms that block re-initiation of DNA synthesis within the same cell cycle.
However, during G2 phase, cell growth, RNA and protein synthesis continue in preparation for mitosis. RNA synthesis occurs throughout interphase as shown by the incubation of cells with 3H Uridine, a precursor of RNA. RNA synthesis, however, stops during mitosis.
The G1 phase corresponds to the interval (gap) between mitosis and initiation of S phase. During G, phase, the cell is metabolically active but does not replicate its DNA. It involves transcription of three types of RNAs, viz., rRNA, tRNA and mRNA.
rRNA synthesis is indicated by the appearance of nucleolus in the interphase nucleus. Proteins synthesized during phase include regulatory proteins, enzyme (e.g., DNA polymerase), tubulin and other mitotic apparatus proteins.
At a point late in G2 phase, all cells follow either one of the two paths. They either withdraw from the cycle and enter a resting phase called G0 stage or they become committed to’ initiate DNA synthesis and thereby completing the cycle.
The G1 is followed by S phase (Fig. 3.31). Histone proteins and their mRNAs are exclusively synthesized during S phase. In all cells, condensed heterochromatic regions of the chromosomes are replicated during S phase. Auto radiographic studies have shown that the centromeric heterochromatins replicate later than rest of the chromosome.
ADVERTISEMENTS:
The duration of different phases of cell cycle varies considerably among organisms, among cells at different steps in the life cycle of an organism, and among different cell types in the same organism. For example, cell cycle of dermal cells in human skin is continuous, other cell types including many nerve cells, withdraw from the G1 phase and enter G0.
Still other cell types such as white blood cells can be recruited to return from G0 and re-enter new cell cycle. For a typical rapidly proliferating human cell with a total cycle time of 24 hours, the G1 phase might last about 11 hours, S phase about 8 hours, G2 phase about 4 hours and M phase about 1 hour.
Cell cycle may range from less than 30 minutes in very rapidly dividing cells of cleaving embryos (other than mammals) to cycles lasting several months in slowly growing tissues, such as the mammalian liver.
Cells at different stages of the cell cycle can also be distinguished by their DNA content. For example, animal cells in G1 phase are diploid with DNA content referred to as 2n. During S phase, replication increases the DNA content from 2n to 4n, the DNA content then remains at 4n for cells in G2 and M phases, decreasing to 2n after cytokinesis.
The DNA content of cells can be experimentally shown by incubating the cells with fluorescent dye that binds to DNA and subsequent analysis of the fluorescence intensity by flow cytometer.
2. Regulation of the Cell Cycle:
The cell cycle of proliferating somatic cells consists of mitotic phase (M) and interphase between divisions. Interphase itself consists of three phases: G1 S and G2. The progression of cell through the division cycle is regulated by extracellular signals from the environment as well as by internal signals that monitor and coordinate various processes that take place during different cell cycle phases.
An important regulatory point that occurs late in G1 and controls progression from G1 to S phase is known as START (in yeast). Once cells have passed START, they are committed to entering S phase and undergoing one cell division cycle.
However, passage through START is regulated by different factors like availability of nutrients, cell size etc. For example, if yeasts are faced with a shortage of nutrients, they arrest their cell cycle at START and enter a resting state rather than proceeding to S phase. START also act as a point at which cell growth is coordinated with DNA replication and cell division.
The proliferation of most animal cells is similarly regulated in the late G, phase of cell cycle, called the restriction point. However, in contrast to yeast, in animal cells, the passage through the restriction point is regulated by extracellular growth factors rather than by availability of nutrients.
In absence of growth factors, progression through the cell cycle stops at restriction point and the arrested cell then enter a quiescent stage of cell cycle called G0, where the cells are metabolically active, but have reduced rates of protein synthesis.
On the other hand, the transition from G2 to M phase is also regulated by extracellular signals in animal cells, e.g., vertebrate oocyte can remain arrested in G2 phase for long period of time until their progression to M phase is triggered by hormonal stimulation.
Cell Cycle Check Points:
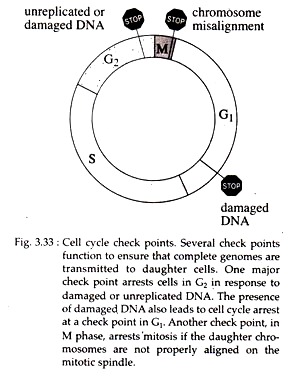
In most cells, the coordination between different phases of the cell cycle is dependent on a system of check points and feedback controls that prevent entry into the next phase of the cell cycle until the events of preceding phase have been completed.
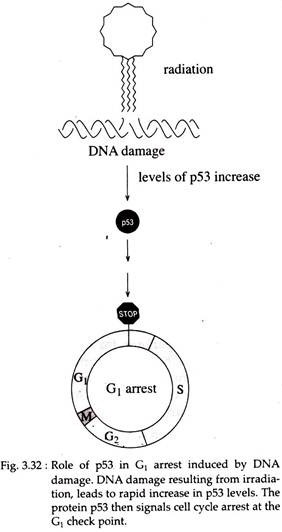
One major check point arrest cells in G2 phase in response to damaged or un-replicated DNA. The presence of damaged DNA also leads to cell cycle arrest at a check point in G1 phase. In mammalian cells, arrest at the G1 check point is mediated by the action of a protein p53 encoded by the tumor suppressor gene p53.
Loss of p53 function due to general mutation prevents G1 arrest in response to DNA damage (Fig. 3.32). Another checkpoint, in M phase, arrests mitosis if the daughter chromosomes are not properly aligned on the mitotic spindle (Fig. 3.33).
3. Regulators of Cell Cycle Progression:
Current studies conducted during the last decade have revealed that the cell cycle of all eukaryotes is controlled by a conserve set of protein kinases, which are responsible for triggering the major cell cycle transitions.
MPF:
The maturation promoting factor or MPF, initially discovered from frog oocytes, appears to be a dimer of cyclin B and the Cdc2 protein kinase and is proved to be a key molecule responsible for regulating the entry of both mitotic and meiotic cells from G2 to M transition in all eukaryotes.
Cyclins and Cyclin-dependent Kinases:
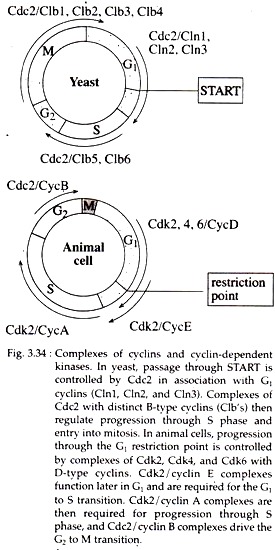
The cell cycles of higher eukaryotes are controlled not only by multiple cyclins, but also by multiple Cdc2 related protein kinases known as Cdks (cyclin-dependent kinases).
The G2 to M transition is driven by Cdc2 in association with the mitotic B-type cyclins (Clbl, Clb2, Clb3 and Clb4). Progression from G1 to S is regulated principally by Cdk2 and Cdk4 (and in some cells Cdk6) in association with cyclins D (cyclin Dl, D2 and D3), E and A (Fig. 3.34).
4. Growth Factors and the D-Type Cyclins:
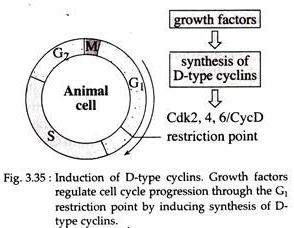
Cyclin-D synthesis is induced in response to growth factor stimulation and the D type cyclins continues to be synthesized as long as growth factors are present. As long as growth factors are present through G1, complexes of Cdk4/Cyclin D drive cells through the restriction point.
If growth factors are removed prior to this key regulatory point in the cell cycle, the levels of cyclin D rapidly fall and cells are unable to progress through G1 to S1 instead becoming quiescent and entering G0 (Fig. 3.35).
Thus, a critical link between growth factor and cell cycle progression is provided by the D-type cyclins. Now it is well known that the tumor suppressor gene Rb (Retinoblastoma) is phosphorylated by Cdk4/Cyclin D complexes that ultimately results in transition from G1 to S phase.
5. Inhibitors of Cell Cycle Progression:
In addition to various growth factors and kinases that regulate cell proliferation, a variety of signals do exist that inhibit cell cycle progression. For example, agents that damage DNA result in cell cycle arrest, and some factors inhibit the proliferation via the induction of Cdk inhibitors.
The best example of action of Cdk inhibitor is provided by cell cycle arrest in response to DNA damage, which is mediated by the protein p53 (see tumor suppressor genes).
The best characterized extracellular inhibitor of animal cell proliferation is TFG-β – a polypeptide that inhibits the proliferation of a variety of epithelial cells in G1. This action of TGF-β appears to be mediated by induction of the Cdk inhibitors p15 and p27, which bind to Cdk4/ Cyclin D complexes. As a result, Rb phosphorylation is blocked and the cell cycle is arrested in G1.
6. Cell Cycle and Cancer – Involvement of Oncogenes and Anti-oncogenes:
The multistep process of cancer is caused by innumerable factors, such as spontaneous genetic changes, exposure to carcinogens or cancer inducing viruses. However, the intrinsic defects in the cell cycle machinery leading to the development of cancer will be briefly discussed here.
Normal healthy eukaryotic cells grow and divide only when the balance of growth stimulatory and growth inhibitory signals received from outside the cell, favour cell proliferation. A neoplastic cell, however, has the lost control of cell division process and reproduces without constraints.
Two classes of genes so far, have been shown to be involved directly in the regulation of cell cycle as well as transformation of a cell to a tumorous state. These are oncogenes and tumor suppressor genes or anti- oncogenes. The products of oncogenes normally stimulate cell proliferation.
Actually most oncogenic products are nothing but different growth factors (e.g., two polypeptides of PDGF [platelet derived growth factor] are encoded by the proto-oncogene sis) and protein kinases (e.g., the protein kinase PP60 src is produced by cellular oncogen src) which are essential for progression of cell through cell cycle.
Now excessive and untimely expression of these oncogenes in cells that do not normally produce the growth factors or kinases will result in uncontrolled and untimely cell proliferation or neoplastic growth.
In normal cells, expression of proto-oncogenes is tightly controlled so that cell growth and division occur only as appropriate for the cell type involved. Several genetic changes like point mutation, deletion, gene amplification and chromosomal translocation can convert the proto-oncogene into oncogene resulting in the loss of that tight control and unscheduled cell proliferation.
In the malignant cells from patients with bladder cancer, lung and colon cancer and leukemia, a single point mutation in codon 12, 13 or 61 in the ras genes has been found.
The effect of this mutation is to cause the G proteins to loose their ability to be regulated so that constitutive growth signals (growth signals that are always being produced) are transmitted into the cell. As a result, unregulated cell proliferation can commence.
Similarly the myc oncogene can arise from its proto-oncogene by deletion and formation of Philadelphia chromosome by reciprocal translocation. All such changes may result in uncontrolled proliferation of cells or neoplastic growth.
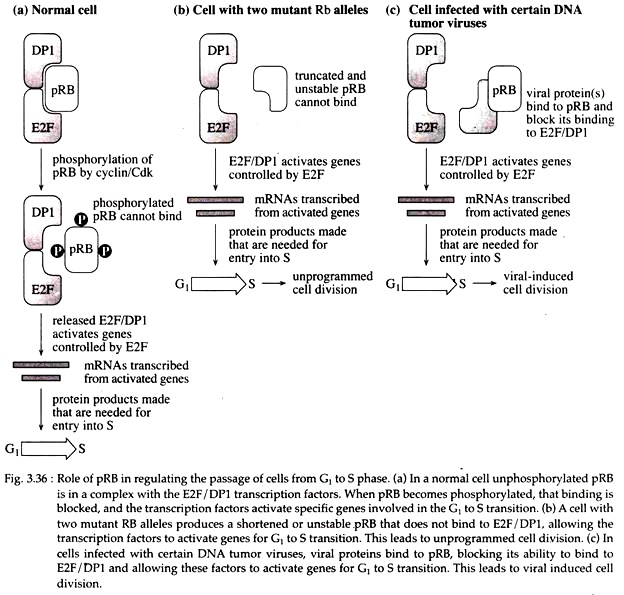
Tumor suppressor genes normally inhibit cell proliferation. The Rb gene is a well-studied tumor suppressor gene located at chromosome 13q14.1 – q14.2 and encodes a 110- kDa nuclear phosphoprotein pRB. It plays a major role in the cell cycle by regulating the passage of cells from G1 to S phase. This transition is important to the cell because it commits the cell to progress through the rest of the cell cycle.
In normal cells or in a cell heterozygous for Rb gene, un-phosphorylated pRB binds to a complex of E2F and DPI transcription factors. When pRB becomes phosphorylated, that binding is blocked, and transcription factors activate specific genes involved in the G1 to S transition.
In normal cells, phosphorylation and dephosphorylation of pRB protein occur very timely. But cells with two mutant Rb alleles produce a shortened or unstable pRB that does not bind to E2F/DP1, allowing the transcription factors to activate genes for G1 to S transition untimely and it leads to un-programmed cell division.
In cells infected with certain DNA tumor viruses, viral proteins bind to pRB, blocking its ability to bind to E2F/DP1 and allowing these factors to activate genes for G1 to S transition and leads to viral induced uncontrolled cell division (Fig. 3.36C) as shown in patients with retinoblastoma.
Another tumor suppressor gene is p53, so named as it encodes a protein of molecular weight 53kDa (called p53). When both alleles are mutated, p53 may be involved in the development of many human cancers including breast, lung, liver, brain, colorectal, bladder and blood cancer.
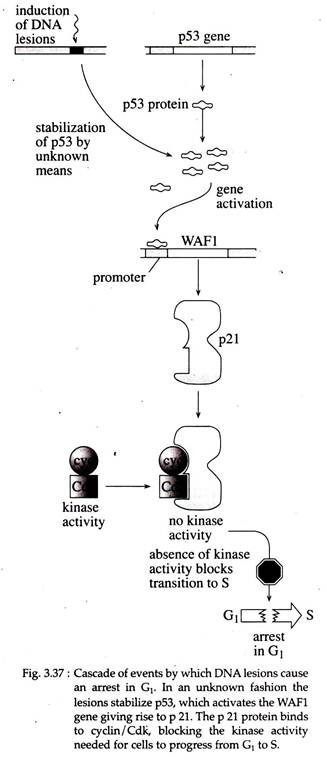
Research indicated that several genes bind p53, one of which — WAF1 is specifically activated by wild type p53. The product of WAF1 is a 21kDa protein called p21, which, when overexpressed, has been shown to cause cells to arrest in G1.
The arrest is caused because p21 binds to cyclin/cyclin dependent kinase (Cdk) complexes and block the activity required to activate the genes needed for the cell to make the transition from G1 to S. In normal cells, in an unknown way DNA damage results in stabilization of p53, and the cascade of events as shown in Fig. 3.37 occur.
The G1 arrest gives the cell time to induce the necessary pathways to repair the DNA damage, after which the cell cycle can resume. If the extent of DNA damage is too great and all lesions cannot be repaired, the cell cycle will not resume, but the damaged cell will die by programmed cell death (apoptosis). Thus the induction of apoptosis is an important function of p53.
But in cells with mutant or inactivated p53 alleles, active p53 is not present. Thus, WAF1 cannot be activated, and no p21 is available to block cyclin dependent kinase activity. So the cell is unable to be arrested in G1. Therefore, the cell cycle may proceed to S. Cells with mutated p53 alleles thus do not undergo apoptosis; instead they accumulate further genetic damage and hence increase the probability of cancer.
In essence, the development of most cancers is a stepwise process involving an accumulation of mutations in a number of genes. The statistics of increasing occurrence of cancers in human, as they age, suggest that perhaps six or seven independent mutations are needed over several decades of life in order for cancer to be induced.
This multiple mutational events typically involve both activation of oncogenes and inactivation of tumor suppressor genes, with a resulting breakdown of the multiple cellular mechanisms that regulate cell cycle and cell differentiation.