In this article we will discuss about the Plasma Membrane:- 1. Meaning of Plasma Membrane 2. Structure of the Plasma Membrane 3. Chemical Composition 4. Molecular Organization 5. Evidences on Fluidity 6. Asymmetry 7. Greater Concept.
Contents:
- Meaning of Plasma Membrane
- Structure of the Plasma Membrane
- Chemical Composition of Plasma Membrane
- Molecular Organization of Plasma Membrane
- Evidences on Fluidity of Membrane Lipids
- Asymmetry in Plasma Membrane Organization
- Greater Plasma Membrane Concept
1. Meaning of Plasma Membrane:
The plasma membrane is an ultra-thin, elastic, living, dynamic and selective- transport barrier, that encloses the content of the entire cell. In both prokaryotic and eukaryotic cells, it physically separates the cytoplasm from the surrounding environment.
ADVERTISEMENTS:
The cells of bacteria and plants have the plasma membrane between the cell wall and the cytoplasm. Plasma membrane forms’ the cell surface for cells without cell wall (e.g., mycoplasma and animal cells).
All biological membranes including the plasma membrane and membranes of other cytoplasmic organelles, e.g., endoplasmic reticulum, Golgi complex, nucleus, mitochondria, chloroplast, lysosomes, peroxisomes etc. are similar in general structure but differ in specific functions.
The plasma membrane is also called cytoplasmic membrane, cell membrane, or plasma lemma. The term plasma membrane has been given by J. Q. Plowe in 1931.
2. Structure of the Plasma Membrane:
ADVERTISEMENTS:
The plasma membrane is so thin that it cannot be observed by the light microscope. In fact, it wasn’t until the late 1950s that techniques for preparing and staining tissues had progressed to the point where the plasma membrane could be clearly resolved in the electron microscope.
In the study of the molecular organisation of the cell membrane, plasma membranes are generally isolated from erythrocytes subjecting them to haemolysis.
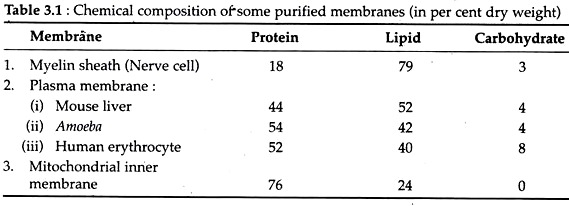
3. Chemical Composition of Plasma Membrane:
Plasma membranes from different sources are found to contain protein, lipids and carbohydrates in different ratios. The wide variations in the lipid-protein carbohydrate ratio between these compounds in different cell membranes are given in Table 3.1.
Membrane Lipids:
The main lipid components of the plasma membrane are phospholipid, cholesterol and galactolipids. The major phospholipids are phosphatidylcholine, phosphatidyl-ethanolamine and sphingomyelin, all of which have no net charge at neutral pH (i.e., neutral phospholipids).
Five to 20 per cent of phospholipids are acidic like phosphatidylinositol, phosphatidylserine, cardiolipin and sulfolipids etc. These are negatively charged and remain associated with proteins by way of lipid- protein interactions. Mammalian cells may contain more than 100 types of phospholipids. Cholesterol is especially abundant in the plasma membrane of mammalian cells but absent in prokaryotic cells and most plant cells.
Membrane Proteins:
Depending on the cell types, a membrane may contain from 12 to more than 50 different proteins.
According to the intimacy of their relationship to the lipid layers, proteins are of three types:
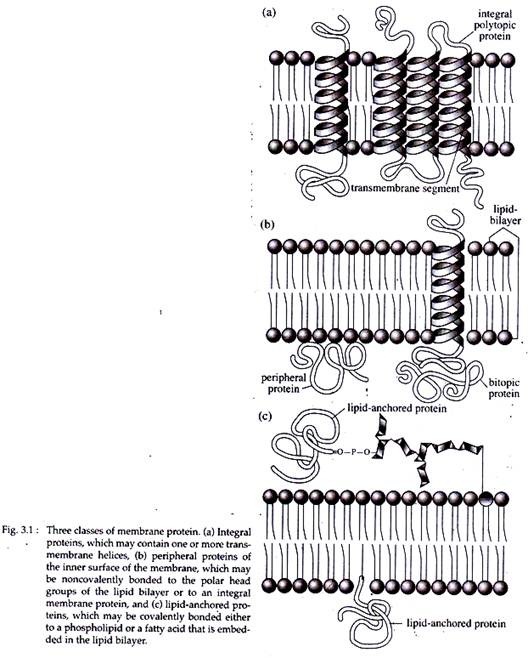
i. Integral Proteins:
These proteins penetrate the lipid bilayer and have domains that protrude from both extracellular and cytoplasmic sides of the membrane. The segment of protein, that pass through the lipid bilayer is called trans membrane segments.
Proteins that possess one trans membrane segment and cross the membrane a single time and are exposed on both sides of the membrane are called bi-topic proteins, e.g., a growth factor, a peptide hormone (Fig. 3.1b).
ADVERTISEMENTS:
Poly-topic proteins possess more than one trans membrane segment and thus weave back and forth across the membrane e.g., the receptor for signal in ER membrane. On the other hand, mono-topic proteins are rare that remain embedded in the lipid bilayer and are exposed at only one membrane surface.
Integral proteins are amphipathic, having both hydrophilic and hydrophobic portions. Hydrophilic parts remain protruded beyond the edge of the bilayer on one or both sides or form an aqueous channel through it. Hydrophobic domains help in the insertion of proteins in the lipid wall.
ii. Peripheral Proteins:
These proteins are located entirely outside the lipid bilayer, on either the extracellular or cytoplasmic surface and are associated with membrane by non-covalent bonds. They can usually be solubilized by extraction with aqueous solution of high salt or alkaline pH.
The best studied peripheral proteins are located on the inner surface of plasma membrane that act as a flexible ‘skeleton’ to provide mechanical support for the membrane during rapid changes in cell shape. Others act as enzymes or factors that transmit trans membrane signals. (Fig. 3.1b)
iii. Lipid-anchored Proteins:
These are located outside the lipid bilayer, but are covalently linked to a lipid. These proteins are of two types. One type present on the external face remains bounded by a short oligosaccharide, linked to a molecule of glycophosphatidylinositol (GPI) that is embedded in the outer leaflet of the lipid bilayer.
Another group of proteins present on the cytosolic face is anchored to the membrane by long hydrocarbon chains embedded in the inner leaflet of the lipid bilayer (Fig. 3.1c).
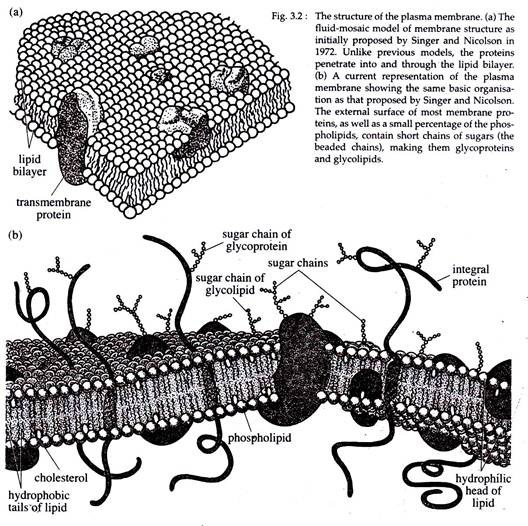
The structure of the plasma membrane:
(a) The fluid-mosaic model of membrane structure as initially proposed by Singer and Nicolson in 1972. Unlike previous models, the proteins penetrate into and through the lipid bilayer.
(b) A current representation of the plasma membrane showing the same basic organization as that proposed by Singer and Nicolson.
The external surface of most membrane proteins, as well as a small percentage of the phospholipids, contains short chains of sugars (the beaded chains), making them glycoproteins and glycolipids.
Membrane Carbohydrates:
In eukaryotic cells, only the plasma membrane contains carbohydrates that are covalently linked to both lipid and protein components, to form glycolipid and glycoproteins, respectively. The carbohydrates of the plasma membrane face outward into the extracellular space (Fig. 3.2b).
In glycoproteins, the carbohydrates are present in short, branched, oligosaccharides, typically haying fewer than about 15 sugars per chain. Sugars commonly found in these carbohydrates are D-glucose, D-mannose, D-galactose, L-fucose, N-Acetyl-D-Glucosamine, and N-Acetyl-D-Galactosamine, etc. Glycolipids function as ‘gates’ for the entrance of many pathogens and as receptor in normal cell function.
4. Molecular Organization of Plasma Membrane:
Many studies have been carried out through years and decades to understand the molecular organization of the plasma membrane. Since it is beyond the resolution of the light microscope, it renders a morphological approach of its study, quite unfeasible with such instrument.
Thus, most of the experimental approaches have been provided only by indirect evidences of the existence of such a membrane around the cells. In the text it has been narrated in brief the evolution of presently well accepted fluid-mosaic model of plasma membrane.
i. Overton’s Concept:
In 1902, Overton postulated that the plasma membrane is composed of a thin layer of lipid.
ii. ‘Lipid bilayer’ Model of Gorter and Grendel:
Gorter and Grendel (1926) found that the lipids extracted from RBC membranes, when suspended in water, spread as a monolayer at the air-water interface and the area occupied by this monolayer was nearly twice the total surface area of the intact RBC itself.
Hence, they concluded that the plasma membrane consists of a lipid bilayer. They further held that the hydrophilic or polar head groups of the lipids face outward while the hydrophobic tails of the lipids face the membrane interior to form a stable membrane. This model is at present given only historical recognition. However, Gorter and Grendel were the first workers to propose that membranes have a lipid bilayer.
iii. Danielle-Davson model:
From experiments on surface tension, Danielle and Davson (1935) found that the surface tension of plasma membrane was lower than pure lipid droplets and that the surface tension of pure lipid droplets could be decreased by addition of proteins to the lipids.
They proposed that the plasma membrane consists of a lipid bilayer sandwiched between two layers of proteins. Thus, the protein layers were held to cover the hydrophilic head groups of the lipids.
This model postulated the presence of both lipids and proteins in membranes. However, subsequent studies revealed that the proteins as well as lipids are not so symmetrically arranged in membranes as was postulated by them.
iv. Unit Membrane Model of Robertson:
The introduction of electron microscopy in biological studies in the early 1950s revealed that the plasma membrane as well as different intracellular membranes, like mitochondrial membrane and the membranes of ER (endoplasmic reticulum) and Golgi complex, all have a trilaminar appearance.
The membrane consisted of a central electron-lucent broad line sandwiched between two thin but electron dense lines. From this data, Robertson (1959) propounded his “Unit membrane hypothesis” in which he stated that all cellular membranes have a similar trilaminar structure consisting of a lipid bilayer sandwiched between two layers of proteins.
Besides, based on the data available from X-ray diffraction studies, he further held that a membrane is about 7.5 nm thick, the lipid bilayer has a thickness of 3.5 nm and each protein layer is 2 nm in thickness.
Moreover, the proteins exist as p-pleated sheets over the hydrophilic heads of lipids. Robertson further proposed that the proteins on two surfaces of lipid bilayer may differ in nature. Thus, he first thought of asymmetric distribution of membrane proteins.
Robertson’s model is practically a reiteration of Danielli-Davson model with extension of that model for all cellular membranes and with some additional idea on the organization of membrane proteins.
However, subsequent studies using optical rotatory dispersion, infrared spectroscopy and circular dichroism revealed that membrane proteins do not exist as P-pleated sheets, but instead, they are mostly globular proteins having a-helical organization.
v. Fluid Mosaic Model of Singer and Nicolson:
The concept of molecular organisation of cell membranes as propounded by Danielli and Davson in the 1930s and Robertson in the 1950s to 1960s underwent significant reorganization following the accumulation of data from enzymatic studies, freeze-fracture studies, ESR (Electron Spin) spectroscopic studies and different other types of sophisticated studies on membranes.
Based on such data, S. Jonathan Singer and Garth Nicolson of the University of California (1972) propounded their ‘Fluid mosacic model’ for membrane organisation. This model had served as the ‘central dogma’ of membrane biology for more than two decades.
The basic postulates of this model are as follows (Fig. 3.2):
(a) The membrane is not a rigid, static structure but a quasi-fluid structure. It is of a fluid consistency like oil. The cellular membrane is a dynamic structure in which the components are mobile and capable of coming together to engage in various types of transient interactions.
The membrane is not just a sandwich of lipid bilayer within two layers of proteins, but, instead, it is a mosaic arrangement of discontinuous protein molecules within lipid bilayer.
On account of its fluidity and the mosaic arrangement of protein molecules, this model of membrane structure is known as the ‘fluid mosaic model’, and it describes both properties as well as organisation of the membrane.
(b) The lipid molecules of the lipid bilayer have their hydrophilic or polar heads facing outward and the hydrophobic tails facing the membrane interior (Fig. 3.2).
(c) Membrane proteins are of two types. Integral proteins remain embedded in the lipid bilayer up to various depths and peripheral proteins remain bound to the outer or inner surface of the lipid bilayer. Some of the integral proteins are trans membrane proteins passing across the lipid bilayer. The peripheral proteins remain associated with membranes by ionic interaction with the hydrophilic heads of the lipid bilayer.
The integral proteins have two parts — the hydrophilic part remains close to the polar head groups of lipid, while the hydrophobic part remains buried in the lipid bilayer due to hydrophobic interaction with the hydrophobic tails of lipids.
(d) Membrane proteins are mostly globular proteins having a-helical organisation instead of being p-pleated sheets.
(e) Both the lipid and integral protein molecules are capable of free movements within the plane of the membrane.
This model has received support from various experimental and analytical studies.
Evidences in favour of Fluid Mosaic Model:
A. Direct evidence from enzymatic studies on mosaic arrangement:
Enzymatic studies by Singer and Nicolson have clearly shown that the proteins do not form continuous layers over the lipid bilayer and instead, may be embedded up to various depths into the lipid bilayer.
Isolated plasma membranes were subjected to hydrolytic action of phospholipase which hydrolyses phospholipids. This enzyme was chosen since the phospholipids constitute the main bulk of the total lipid fraction of most membranes.
Nearly 60-70% of total membrane lipids were removed from the membranes and passed into the medium. The protein fraction of membranes remained unaffected and could not be detected in the medium.
That a major fraction of the membrane- lipids was extracted by the hydrolytic action of the enzyme confirms that the lipid bilayer was not completely covered by protein layers. In other words, the protein molecules remain embedded in the lipid bilayer instead of forming continuous sheaths on the surfaces of the lipid bilayer.
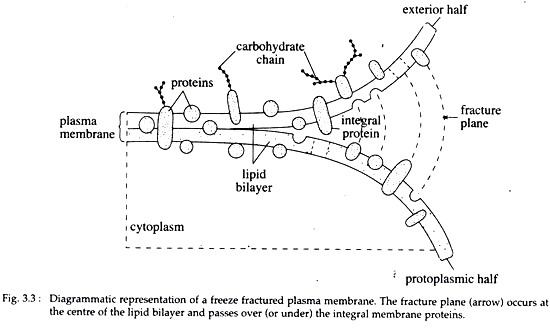
B. Freeze fracture studies of membranes:
Direct evidence in favour of the fluid mosaic model comes from freeze fracture studies of the membrane. This technique not only reveals that the lipid bilayer is not sandwiched between two continuous layers of proteins, but also reveals the asymmetric distribution of membrane proteins as well as mobility of membrane proteins.
Under EM, a replica produced from freeze-fractured RBC ghosts revealed that numerous protrusions are randomly spread on the replica. The protrusions represent the locations of membrane proteins that remained embedded in the lipid bilayer.
The protrusions were more numerous in the inner or protoplasmic half or P-face of the membrane than in the external half or E-face of the membrane. This study clearly indicates that protein particles remain embedded in the lipid bilayer of membranes and are asymmetrically distributed in it (Fig. 3.3).
5. Evidences on Fluidity of Membrane Lipids:
Different experimental studies confirmed the mobility of both membrane lipids and membrane proteins.
i. Fluidity or Mobility of Membrane Lipids:
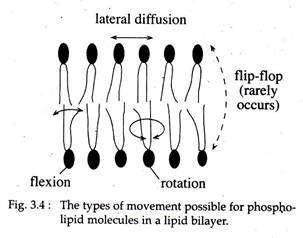
Studies based on biophysical techniques like ‘ESR Spectroscopy’ and ‘Differential scanning colorimetry’ revealed that membrane lipids are capable of two kinds of movements:
(a) Lateral movements (diffusion) within the respective monolayers and
(b) Flip-flop movement or trans-bilayer movement where lipids move across the bilayer.
Lateral movements of lipid molecule are quite fast than the flip-flop movement (Fig. 3.4). Individual lipid molecules can rotate very rapidly around their own axis and their hydrocarbon chains show flexion.
In low temperature, the lipid molecules become immobile, since their hydrocarbon chains remain in a ‘gel state’ or rigid ‘crystalline state’. But at physiological temperature, the lipid molecules become mobile due to rotation of carbon-carbon bonds caused by heat energy.
ii. Fluidity or Movements of Membrane Proteins:
Various experimental studies have confirmed that the membrane proteins are actually capable of lateral movements or lateral diffusions within the lipid bilayer.
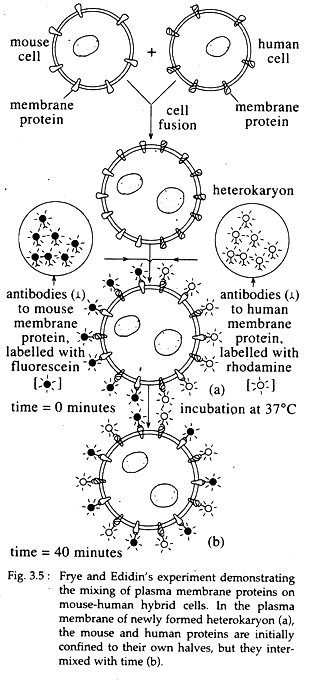
“Cell fusion” Experiment (Frye and Edidin, 1970):
In this experiment, particular membrane proteins of mouse fibroblast cells were labelled with specific antibodies linked with the fluorescent dye fluorescein which gives a green fluorescence. Particular membrane proteins of human fibroblast cells were labelled with specific antibodies linked with the fluorescent dye, rhodamine, which gives a red fluorescence.
The labelled mouse cells and human cells were then induced to fuse together in vitro by treatment with Sendai virus. The resultant fused cells or hybrid cells were kept under incubation at 37°C and observed under the fluorescence microscope.
The surface of the fused cells showed distinct regions of green’ and red fluorescence, indicating distinct locations of mouse and human membrane proteins (Fig. 3.5a). But when the fused cells were observed after 40 minutes of cell fusion, the green and red fluorescing areas were completely intermixed over the surface of the fused cells (Fig. 3.5b).
The results clearly indicate that the mouse and human membrane proteins were able to move laterally along the plane of the fused cell membrane. In other words, intrinsic membrane proteins are capable of lateral diffusion movements through the lipid bilayer, which adds support to the fluid mosaic model of membranes (Fig. 3.5).
Importance of Membrane Fluidity:
Membrane fluidity seems to provide a perfect compromise between a rigid, ordered- structure in which mobility would be absent and a completely fluid, non-viscous liquid in which the components of the membrane could not be oriented.
Fluidity allows for interactions to take place within the membrane, e.g., cluster of membrane proteins to assemble at particular sites with the membrane to form specialized structures, such as intercellular junction, light-capturing complexes and synapses.
Many of basic cellular processes, including Cell movement, cell growth, cell division, secretion and endocytosis depend on the movement of membrane components and would probably not be possible if membranes were rigid and non- fluid in structure.
6. Asymmetry in Plasma Membrane Organization:
At present, it has been confirmed that the lipids, proteins as well as carbohydrates are asymmetrically distributed in the membrane instead of having a simple and symmetrical distribution. This asymmetrical molecular organization has evolved to satisfy the functional properties of the membrane.
i. Asymmetry of Membrane Lipids:
The amount of lipid in plasma membrane may be as low as 20% in bacterial cell membrane and as high as 80% in nerve cell membrane. Different lipid molecules have been found to be asymmetrically distributed in the membrane.
Phospholipids with positively charged heads are located in the outer leaflet of the lipid bilayer whereas those with negative heads are located in the inner or cytoplasmic leaflet of the lipid bilayer. Glycolipids are present only in the outer leaflet.
The functional properties of the membrane may be determined by this lipid asymmetry. For instance, the binding of protein- kinase to the inner face of the membrane is facilitated by the negatively charged head groups of phospholipids in the inner leaflet of lipid bilayer.
ii. Asymmetry of Membrane Proteins:
Membrane proteins display a remarkable asymmetric distribution in the membranes. The asymmetrical distribution of proteins in the two halves or leaflets of the membrane has been called as ‘sidedness’ of membrane proteins.
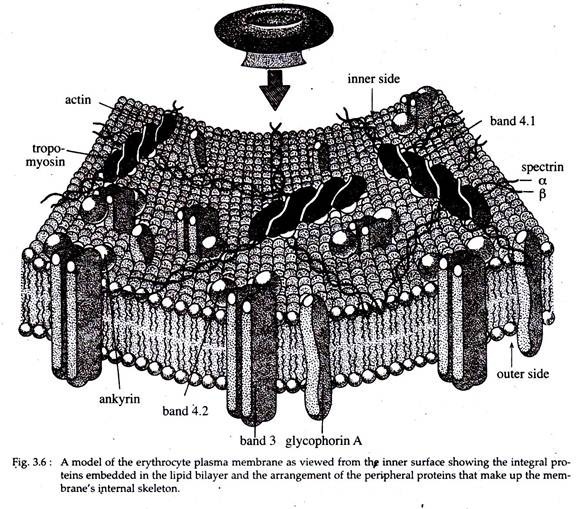
In RBC membrane, it has been found that two proteins, viz., band three protein and glycophorin occur as trans membrane proteins with a part of their molecules exposed on either face of the lipid bilayer.
The enzymatic proteins like acetyl-cholinesterase and NADase lie on the outer face of the lipid bilayer while the cytoskeletal proteins like actin, ankyrin, spectrin etc. and certain enzymes like ATPase and protein kinase lie at the inner surface of the lipid bilayer.
It is suggested that cytoskeletal proteins interacting with the integral proteins maintain cell shape and elasticity while enzymes lying on the outer membrane surface can easily act on extracellular substrates (Fig. 3.6).
iii. Asymmetry of Membrane Carbohydrates:
The carbohydrates are asymmetrically distributed with a remarkable ‘sidedness’. They are found only on the external surface of the membrane. The attachment of carbohydrates renders the status of glycolipids and glycoproteins to those lipids and proteins (Fig. 3.2).
7. Greater Plasma Membrane Concept:
This concept is rather an elaboration of fluid mosaic model than a new model of membrane structure. This concept holds that the plasma membrane has a far more complex organization than a simple lipoprotein framework.
An extraneous carbohydrate coat remains attached to the lipoprotein framework that exists as a ‘greater membrane’ and extends from the exterior environment of a cell up to considerable depth into the cytoplasm of the cell.
The extraneous carbohydrate coat has been variously named as ‘glycocalyx’, ‘sweet coat’ or ‘sweet husk’. In animal cells, the carbohydrates occur as oligosaccharide and polysaccharide chains that remain attached to the membrane lipids or proteins to render glycolipid or glycoprotein nature to the latter molecules (Fig. 3.2).