In this article we will discuss about the Diseases of Silkworm:- 1. Protozoan Diseases 2. Bacterial Diseases 3. Viral Diseases 4. Fungal Diseases.
Protozoan Diseases:
Pebrine:
The most virulent protozoan disease of silkworm is Pebrine, so named as the infected worms show some pepper-like black spots.
Causative organisms:
ADVERTISEMENTS:
Pebrine is caused by the protozoan Nosema bombycis that has several strains of which most virulents are NIK-2r. NIK-3h and NIK-4m in India. The protozoa passes its life cycle through 2 stages. The infective spore stage and vegetative stage.
Modes of transmission:
The disease is transmitted to silkworm in three different ways:
(a) Oral:
ADVERTISEMENTS:
Spores liberated through the faeces of infected worms or through dead larvae, contaminate the mulberry leaves given in rearing bed. Such leaves when taken by other larvae, they get infected.
Again, eggs may be contaminated by such faecal matter or dead tissues containing the spores during the time of oviposition or after oviposition. The newly hatched larvae who take part of chorion of such contaminated eggs may get infection.
(b) Contact:
Larvae may get infection through their skin from the rearing bed contaminated with Nosema spore containing faecal matter or dead tissues.
ADVERTISEMENTS:
(c) Transovarial:
If infection occurs in 5th instar stage, the adult moth normally emerges through pupal stage. However, the spores of Nosema ingested then sporulate within the oocyte and pass on to the eggs. Thus, the egg itself acquires infection from the layers (mother) and hatches into primary infected larvae.
However, these infected eggs hatch in very low percentage and the hatched out larvae usually die before reaching 3rd instar. These larvae act as the source of secondary infection for other larvae through their faeces or dead tissues.
These larvae, if spin, form weak cocoons and exhibit pebrine symptoms and may die during or after spinning. Thus, they serve as source of tertiary infection to other 5th instar larvae which may spin and may even live up to the adult stage.
Symptoms:
Egg:
Infected eggs do not show firm attachment with the egg card due to improper deposition of glue. These eggs are pale yellow in colour and may fail to hatch.
Larvae:
Primary infected larvae usually die before exhibiting any typical symptoms. Larvae having secondary or tertiary infections may show- different symptoms like loss of appetite, ‘unequals’ appearance due to slow and irregular growth; clean worm symptom that arises due to irregular and incomplete moulting, black pepper-like spots on the body (Fig. 3.36), irregular brown patches due to dead hypodermal cells, spitting and wasting silk instead of spinning the cocoon, etc.
ADVERTISEMENTS:
Infected larvae also pass soft faeces and often die after spinning without pupating.
Pupa:
Live infected pupa if present inside the cocoon, may show black, swollen body with black spots on sides of abdomen.
Adult moth:
Pebrine infected adult moths show black spot on abdomen, deformed antennae, unstretched, and discoloured wings. Females usually lay eggs in irregular, loose heaps. Scales fall off easily from the body of pebrine infected moths.
Detection of pebrine:
Pebrine at any stage of silkworm life cycle can be detected by observing the above mentioned symptoms. Besides, microscopic observation of homogenate / fluid from dead tissues, faecal pellets can indicate the presence of infective spores of Nosema. Again, by advanced immune enzymatic methods, presence of pebrine spores can be detected quickly.
Control measures:
(i) In grainages only disease- free layings (DFLs) will be allowed for rearing.
(ii) Diseased larvae if detected in rearing tray/bed should immediately be removed and burnt. All rearing appliances including the rearing room then should be disinfected with 4-5% formalin solution or by spreading bleaching powder,
(iii) Adult moths showing symptoms of pebrine should not be undertaken for any sericultural processes.
(iv) For disinfection of rearing accessories, other agents like Benomyl. Bavistin, Bengard, etc. can be used instead of routine formalin/bleaching powder.
(v) Rearing of disease resistant races of Bombyx, e.g., Nistari can be considered as an important preventive measure against pebrine.
Bacterial Diseases:
In sericulture, silkworm can be attacked by various bacteria resulting in different diseases.
Most common bacterial diseases are:
(A) Bacterial flacherie:
The diseases having symptoms of diarrhoea and vomiting are collectively called Flacherie. Again, symptoms of flacherie may not be same in all types of flacherie. The disease is also known as shrinking disease /clear head / faecal chain disease.
Causative organisms:
Bacterial flacherie is caused by Bacillus bombycis, B. sotto, Streptococcus bombycis, Pseudomonas aeroginosa, etc. In general, the pathogenic bacteria may attack their alimentary canal and haemolymph.
Modes of transmission:
(a) Oral:
Larvae when take contaminated leaves can get infection directly. The entered bacteria attack and grow in their alimentary canal. Due to bacterial growth and consequent accumulation of lactate, acetate, etc. the permeability of gut is lowered due to lowering of pH.
Then the bacteria become able to invade the haemocoel and spread the infection in whole body of larvae. The infected larvae pass bacterial spore containing stool which acts as new source of infection.
(b) Bad rearing condition:
Temperature and humidity if not maintained properly in the rearing room, may enhance the growth and spreading of flacherie bacteria.
Symptoms:
The diseased silkworms show following general symptoms:
(i) Lack of appetite,
(ii) Stunted growth,
(iii) Sluggish movements,
(iv) Soft and loose skin (Fig. 3.37a),
(v) Shrinkage of body after moulting and these larvae usually refuse to take any food,
(vi) The body become dull coloured to black-brown,
(vii) The larvae show signs of pain, convulsions and die,
(viii) The carcass turns black and emits foul smell (Fig. 3.37b),
(ix) Diarrhoea and vomiting.
Detection of bacterial flacherie:
Above mentioned symptoms help to detect about flacherie – infected larvae. Besides, histopathological studies can reveal affected cylindrical cells in gut wall in case of streptococcus infection and affected goblet cell in case of Diplococcus infection.
Control measures:
(i) Optimum environmental conditions with required temperature and humidity should be maintained in the rearing place,
(ii) Healthy and quality leaves should be supplied to rearing larvae,
(iii) Unhealthy larvae should be discarded from rearing trays, and
(iv) Regular disinfection of rearing room and appliances should be done with 2% formalin.
(B) Septicemia:
It is resulted following infection of haemolymph by bacteria.
Causative organism:
Septicemia mainly caused by Bacillus, Streptococcus and Staphylococcus.
Modes of transmission:
(i) Contact through wounds on skin, and
(ii) Oral route or intake of contaminated food can also transmit the infection.
Symptoms:
(i) Softening of body,
(ii) Change of body colour to brown,
(iii) Liquid excreta,
(iv) Loss of clasping power of prolegs and
(iv) Death of larvae.
Control measures:
(i) Good sanitation and healthy environment should be provided,
(ii) Infected larvae should be removed/discarded,
(iii) Larvae should be handled carefully to reduce skin wounds.
(C) Sotto disease:
This disease is caused by the toxins released by some bioinsecticidal bacteria.
Causative organism:
Bacillus thuringiensis, bacteria widely used as a bioinsecticide, release some endotoxin thuricide, that remains stored in a parasporal crystalline structure during the time of sporulation of the bacteria.
This toxin, if taken by the larvae orally causes two types of responses:
In type I response, the larvae of B. mori stop feeding, haemolymph becomes alkaline and paralysis occurs within 60-80 minutes of intake. In type II response, other lepidopteran larvae only show rapid inhibition of feeding.
Mode of transmission:
Oral intake of mulberry leaves contaminated with toxin-crystals can cause toxicity to the larvae. But if infected by injection to the larvae, the same bacterial toxins show no effects to the silk worm larvae.
Symptoms:
(i) Lack of appetite,
(ii) Sluggish movement,
(iii) Shrinkage of skin,
(iv) Diarrhoea,
(v) Loss of clasping power of prolegs, and
(vi) Death. After death the body becomes black in colour, internal organs become liquid and emit foul odour.
Control measures:
(i) Good sanitation, specially cleaning the room with hot water to inactivate the toxin,
(ii) Removal of dead / infected larvae from rearing tray,
(iii) Bacterial spores if detected can be destroyed by exposing to 2% formaldehyde for 3 hours or to 100°C for 5 minutes.
(D) Court disease:
This bacterial disease is also known as Rangi in India because of the colours shown by dead larvae.
Causative organism:
In this disease, both larvae and pupae get infected by the bacteria Serratia marcescans and also by S. piscavotar following the primary infection with Streptococcus faecalis.
Mode of transmission:
(i) Through skin wounds and
(ii) oral route.
Symptoms:
(i) Affected larvae are flaccid
(ii) Death, and
(iii) Following death, the body colour of larvae changes to crimson red.
Control measures:
(i) Hygienic and clean rearing,
(ii) Isolation and killing of diseased larvae and pupae and
(iii) Disinfection of rearing place and appliances with 2% formalin.
Viral Diseases:
(A) Grasserie:
The complex of diseases caused by viruses showing jaundice-like symptoms are collectively referred to as grasserie or polyhedrosis.
According to the kind of causative viruses, location of the latter in host’s tissue and cell, polyhedrosis again are of 2 types:
(1) Nuclear polyhedrosis which according to recent concept is considered as grasserie and
(2) Midgut cytoplasmic polyhedrosis or Midgut nuclear polyhedrosis.
Causative organisms:
The nuclear polyhedrosis of B. mori is caused by Nuclear Polyhedrosis Virus strain Bm (NPV Bm). This DNA virus multiplies only in the nucleus of the host cells, where it forms hexagonal polyhedra which remain embedded in a proteinaceous matrix, called Polyhedral Inclusion Bodies (PIB).
The inclusions of PIB are synthesised by the silkworm (host) machinery, independent of viral components and so, may or may not contain viral particles. In midgut polyhedrosis, the RNA Smithia virus forms polyhedra in cytoplasm in case of midgut cytoplasmic polyhedrosis or in nucleus in case of midgut nuclear polyhedrosis.
Modes of transmission:
Nuclear polyhedrosis can be transmitted to healthy worms from infected worms by the following routes:
(i) Oral:
Ingestion of leaves contaminated with PIB from the dead tissue or faeces from infected worms can transmit the disease to fresh worms.
(ii) Environment:
High temperature, humidity, etc. may induce the spreading of this disease by transforming latent stage to virulent stage of the pathogen.
(iii) Blockage of spiracles:
Contaminated leaves with formalin, exposure to camphor during rearing and blocking of spiracles by dust, Uzi fly, etc. may induce the disease.
(iv) Skin wound:
Skin wound may get infection from contaminated rearing bed.
Midgut polyhedrosis, on the other hand, can be transmitted through oral route or by induction due to bad environment.
Symptoms:
In case of nuclear polyhedrosis, the late stage of infection shows the following symptoms:
(i) Loss of appetite,
(ii) Loose and shiny white skin with elevated protuberances in intersegmental zones,
(iii) Ruptured skin exuding milky-white or yellowish fluid containing polyhedra (Fig. 3.38).
(iv) Restless larvae usually try to leave the rearing tray and die due to exuding body fluid,
(v) Infected larvae usually do not moult,
(vi) Body colour turns to yellow and faeces are white in colour because of accumulated polyhedra, so the name ‘jaundice’ is given to the disease.
For midgut viral infection, the worms show initially no symptoms.
The symptoms appear only in late stage and include:
(i) Loss of appetite,
(ii) Translucent cephalothorax and shrinkage of body,
(iii) Reduced growth,
(iv) Opaque midgut due to accumulation of polyhedra in cytoplasm,
(v) White, loose faeces due to accumulation of polyhedra, and
(vi) Irregular moults etc.
Control measures:
(i) Rearing environment should be hygienic with proper ventilation,
(ii) Sterilisation of rearing room with steam or 2% formalin or bleaching powder or Resham Keed Ouzhad (RKO) or Labex,
(iii) Removal of dead or infected worms from rearing tray
(iv) Feeding the larvae with healthy leaves, and
(v) Oral administration of nalidixic acid, P-aminobutyric acid etc. or topical application of imanine can control NPV to some extent.
(B) Infectious flacherie:
Besides bacteria, Infectious Flacherie Virus (IFV), an RNA virus can also cause flacherie in the host silkworm where it forms irregular inclusions mostly in the midgut goblet cells.
Modes of transmission:
The causative virus enter the host midgut through oral route via ingested contaminated leaves.
Symptoms:
(i) Shrinkage of body with transparent appearance.
(ii) Vomiting of digestive juice.
Control measures:
(i) Feeding the larvae with healthy leaves,
(ii) Hygienic rearing, and
(iii) Disinfection of rearing room and appliances.
(C) Gattine:
This disease is also known as clear head disease because of the transparent appearance of affected worms.
Causative agents:
Primary agent is some virus while Streptococcus bombycis acts as the secondary invader.
Symptoms:
Following secondary infections by bacteria the larvae show:
(i) Lack of appetite,
(ii) vomiting of an alkaline, clear fluid, and
(iii) the anterior part of the alimentary canal appears transparent due to absence of any mulberry leaves and hence given the name of “Clear Head” to this disease.
Control measures:
(i) Rearing of larvae in hygienic conditions, and
(ii) Disinfection of rearing place and appliances between each stage of whole rearing process.
Fungal Diseases:
(A) Muscardine:
Fungal diseases of silkworm are called muscardine where the body of larvae gets mummified due to deposition of calcium oxalate and hence the disease is also called ‘calcino’.
Causative organisms:
Different fungi cause this disease but the name varies according to the colour of conidiospores, produced by them on the body of worms. Name of different muscardines and their causative fungi are given below.
White muscardine is caused by Beauveria bas- siana where the conidia are spherical/oval and spores are like white powdery specks. Black muscardine is spread by Metarrhizium arisopliae. The conidia are cylindrical and the spores are dark greenish black in colour.
The fungus Poecilomyces farinosus causes yellow muscardine, where oval to spherical spores collectively look yellow in colour. Aspergillus flavus and A. oryzel cause brown muscardine with producing spores dirty brown in colour. Red muscardine may be caused bv Sorosporella uvella and Spicaria fumosorosea with their red spores.
Modes of transmission:
Contact:
All types of muscardine infections are transmitted through skin. However, oral transmission through ingestion of contaminated leaves and transmission through spiracles are also noticed. The fungal spores upon falling on the body surface of silkworm germinate and gradually penetrate the cuticle by mechanical and enzymatic forces. The growing hyphae then draw nourishment from host’s tissue and ultimately kill them.
The fungi then take saprophytic nutrition from the dead larvae tissue and grow mycelia as white flakes over the dead tissue. Reproductive conidiophores with characteristic colour then grow from the mycelia. Due to continuous growth of fungi-colony, and their release of calcium oxalate as metabolite, the dead tissue of host larvae becomes hard and mummified.
Yellow muscardine fungi affect the gut of larvae, while red muscardine fungi attack the body wall. In case of white, green and black muscardines, the pathogenic fungi reach the haemocoel where they spread their hyphae.
Symptoms:
(i) Sluggish movement showing “unequals” in rearing bed,
(ii) Loss of appetite,
(iii) Coloured patches may appear around spiracles or at leg bases,
(iv) Body shrinks with loose cuticles,
(v) Vomiting,
(vi) Early infected larvae do not spin, but late infected instars may spin flimsy cocoon within which the pupae die, and
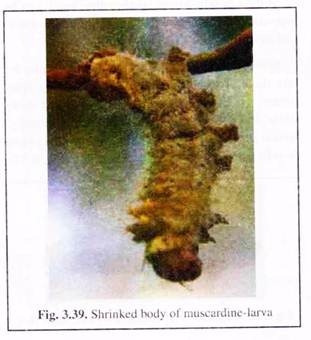
(vii) Dead larvae may be covered with coloured spores of causative fungi or may be mummified into chalky white structure (Fig. 3.39).
Control measures:
(i) Proper rearing:
Rearing room and bed should be free from humidity and darkness as these can stimulate the growth of fungi. Free aeration by keeping windows and doors open or by using fan for continuous flow of air should be maintained. Temperature should be kept above 22°C. Any kind of dampness in and around rearing place is to be avoided.
For this purpose, anhydrated lime can be kept in the corner of rearing room. All rearing appliances along with leaf-food should be kept free from extra moisture. During moulting, new bed should be kept thin and old newspaper can be kept below the bed to prevent muscardine infection during wet season.
(ii) Disinfection:
For preparing disinfected rearing beds, 0.4% formalinised husk can be used for the beds of I and II instars; 0.5% for II and III instars, 0.6% for IV and 0.8% for V instar. The ratio of diluted formalin and burnt husk is usually kept 1:10 (v/v).
After each bed cleaning and during moulting, a mixture of dithane-M45 and kaoline can be applied on silkworm to prevent the germination of fungal spores if present on body surface of larvae. Again, for every 100 layings, 3-4 kg of Resham Keed Ouzhad (RKO) can be spread on the worms after each moulting and 30 minutes before the larvae resume feeding.
Labex, a mixture of lime and bleaching powder has anti-muscardine and grasserie effects and can improve larval growth and commercial characters as well. This mixture taken in muslin cloth can be spread directly on silkworm in each instar at a recommended dosage of 4 gm/ 0.1 nr of rearing tray.
Other chemicals used as contact disinfectants for muscardine include benzoic acid, aliband, chemi-chlon, benzoalkonium chloride, mixture of ceresan and lime, etc. Burning of 20-30 gm of sulphur/m3 area of rearing space is very effective to prevent growth of muscardine fungi.
(iii) Removal of infected larvae:
Infected larvae if detected should be removed immediately from rearing tray before the formation of conidiospores on them. Infected larvae may be kept in 2% formalin to kill the spores or buried in deep pits.
(iv) Cleaning the rearing bed:
Litter from the infected bed should be removed immediately after detection of infection and be dumped in deep pits and burnt.
(v) Care in between rearing:
The rearing room and appliances should be disinfected immediately after muscardine infection before the next rearing. These should also be kept dry during the period between rearing.